Abstract
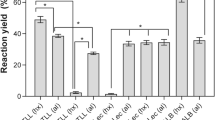
The objective of this work was to assess the influence of compressed propane treatment on the hydrolytic activity of three lipases (Amano PS, Amano AY30, and a non-commercial lipase from Yarrowia lipolytica) free, resuspended, and in immobilized form. To evaluate the effect of process variables on the lipase activity, a semi-factorial experimental design with two levels and four variables was employed for free and immobilized lipases and a full 22 experimental design was carried out for lipases in solution. The residual activity was defined as the ratio of lipase activity before and after treatment with pressurized propane. For free and immobilized lipases, an enhancement in residual lipase activity in most of the experimental conditions investigated was observed. In the case of resuspended lipases, it is shown that enzyme kinetics is sensitive to treatment with compressed propane resulting in remarkable gains and losses of enzyme activity. In a general way, the results showed that the enzyme activity changes significantly depending on the enzyme, the presentation form, and the experimental conditions investigated, allowing the selection of operational conditions in terms of temperature, pressure, exposure time, and depressurization rate for advantageous application of these biocatalysts in hydrolysis reactions.








Similar content being viewed by others
References
Andrade, J. M., Oestreicher, E. G., Antunes, O. A. C., Oliveira, J. V., Oliveira, D., Soares, C. M, & Dariva, C. (2008). Effect of treatment with compressed CO2 and propane on D-hydantoinase activity. Journal of Supercritical Fluids, in press. DOI 10.1016/j.supflu.2007.11.019.
Cernia, E., Palocci, C., & Soro, S. (1998). The role of the reation medium in lipase-catalyzed esterifications and transesterifications. Chemistry and Physics of Lipids, 93, 157–164.
Chaudhary, A. J., Kamat, S., Beckman, E. J., Nurok, N., Hajdu, P., Kleyle, R., & Russell, A. J. (1996). Control of subtilisin substrate specificity by solvent engineering in organic solvents and supercritical fluoroform. Journal of American Chemical Society, 118, 12891–12896.
Chen, J. S., Balaban, M. O., Wei, C. L., Marshall, M. R., & Hsu, W. Y. (1992). Inactivation of polyphenol oxidase by high-pressure carbon dioxide. Journal of Agricultural and Food Chemistry, 40, 2345–2350.
Destain, J., Roblain, D., & Thonart, P. (1997). Improvement of lipase production from Yarrowia lipolytica. Biotechnology Letters, 19, 105–108.
Erbeldinger, M., Mesiano, A. J., & Russell, A. J. (2000). Enzymatic catalysis of formation of Z-aspartame in ionic liquid - an alternative to enzymatic catalysis in organic solvents. Biotechnology Progress, 16, 1129–1134.
Freire, D. M. G., Gomes, P. M., Bom, E. P. S., & Sant’Anna Jr., G. L. (1997). Lipase production by a new promissing strain of Penicillium restrictum. Revista de Microbiologia, 28, 6–12.
Fricks, A. T., Souza, D. P. B., Oestreicher, E. G., Antunes, O. A. C., Girardi, J. S., Oliveira, D., & Dariva, C. (2006). Evaluation of radish (Raphanus Sativus L.) Peroxidase activity after high-pressure treatment with carbon dioxide. Journal of Supercritical Fluids, 38, 347–353.
Fukuda, H., Kondo, A., & Noda, H. (2001). Biodiesel fuel production by transesterification of oils. Journal of Bioscience and Bioengineering, 92, 405–412.
Giβauf, A., & Gamse, T. (2000). A simple process for increasing the specific activity of porcine pancreatic lipase by supercritical carbon dioxide treatment. Journal of Molecular Catalysis B: Enzymatic, 9, 57–64.
Habulin, M., & Knez, Z. (2001). Activity and stability of lipases from different sources in supercritical carbon dioxide and near-critical propane. Journal of Chemical Technology and Biotechnology, 76, 1260–1265.
Heremans, K., Goossens, K., & Smeller, L. (1996). Pressure-tuning spectroscopy of proteins: Fourier transform infrared studies in the diamond. Oxford, USA: Anvil Cell.
Houde, A., Kademi, A., & Leblanc, D. (2004). Lipases and their industrial applications. Applied Biochemistry and Biotechnology, 118, 155–170.
Ishikawa, H., Shimoda, M., Yonekura, A., & Osajima, Y. (1996). Inactivation of enzymes and decomposition of a-helix structure by supercritical carbon dioxide microbubble method. Journal of Agricultural and Food Chemistry, 44, 2646–2650.
Jessop, P. G., & Leitner, W. (1999). Chemical synthesis using supercritical fluids. Weinheim, USA: Wiley-VCH.
Kaewthong, W., & H-Kittikun, A. (2004). Glycerolysis of palm olein by immobilized lipase PS in organic solvents. Enzyme and Microbial Technology, 35, 218–223.
Kamat, S., Barrera, J., Beckman, E. J., & Russell, A. J. (1992). Biocatalytic synthesis of acrylates in organic solvents and supercritical fluids: I. Optimization of enzyme environments. Biotechnology and Bioengineering, 40, 158–164.
Kamat, S., Beckman, E. J., & Russell, A. J. (1993). Control of enzyme enantioselectivity with pressure changes in supercritical fluoroform. Journal of American Chemical Society, 115, 8845–8851.
Kamat, S., Beckman, E. J., & Russel, A. J. (1995). Enzyme activity in supercritical fluids. Critical Reviews in Biotechnology, 15, 41–40.
Kasche, V., Schlothauer, R., & Brunner, G. (1988). Enzyme denaturation in supercritical CO2: stabilyzing effect of S–S bonds during the depressurization step. Biotechnology Letters, 10, 569–573.
Kim, K. W., Song, B., Choi, M. Y., & Kim, M. J. (2001). Biocatalysis in ionic liquids: Markedly enhanced enantioselectivity of lipase. Organic Letters, 10, 1507–1512.
Knez, Z., & Habulin, M. (2002). Compressed gases as alternative enzymatic-reaction solvents: A short review. Journal of Supercritical Fluids, 23, 29–34.
Kumar, R., Madras, S., & Modak, J. (2004). Enzymatic synthesis of ethyl palmitate in supercritical carbon dioxide. Industrial Engineering and Chemical Research, 43, 1568–1573.
Laane, C., Boeren, S., Hilhorst, R., & Veeger, C. (1987). Optimization of biocatalysis in organic media. In C. Laane, J. Tramper, & M. D. Lilly (Eds.), Biocatalysis in organic media (pp. 65–84). Amsterdam, The Netherlands: Elsevier Science.
Laane, C., Boeren, S., Vos, K., & Veeger, C. (2004). Rules for optimization of biocatalysis in organic solvents. Biotechnology and Bioengineering, 30(1), 81–87.
Lanza, M., Priamo, W. L., Oliveira, J. V., Dariva, C. E., & Oliveira, D. (2005). The effect of temperature, pressure, exposure time and depressurization rate on lipase activity in SCCO2. Applied Biochemistry and Biotechnology, 113, 181–188.
Lau, R. M., Rantwijk, F. V., Seddon, K. R., & Sheldon, R. A. (2000). Lipase-catalyzed reactions in ionic liquids. Organic Letters, 26, 4189–4196.
Lou, W. Y., Zong, M. H., Liu, Y. Y., & Wang, J. F. (2006). Efficient enantioselective hydrolysis of D-L phenilglycine methyl ester catalyzed by immobilized Candida antarctica lipase B in ionic liquid containing systems. Journal of Biotechnology, 125, 64–69.
Montgomery, D. C. (1991). Design and analysis of experiments. New York, USA: Wiley.
Nakaya, H., Miyawaki, O., & Nakamura, G. (2001). Determination of log P for pressurized carbon dioxide and its characterization as a medium for enzyme reaction. Enzyme and Microbial Technology, 28, 176–180.
Ndiaye, P. M., Franceschi, E., Oliveira, D., Dariva, C., Tavares, F. W., & Oliveira, J. V. (2006). Phase behavior of soybean oil, castor oil and their fatty acid ethyl esters in carbon dioxide at high pressures. Journal of Supercritical Fluids, 37, 29–33.
Oliveira, D., Feihrmann, A. C., Dariva, C., Cunha, A. G., Bevilaqua, J. V., Destain, J., Oliveira, J. V., & Freire, D. M. G. (2006a). Influence of compressed fluids treatment on the activity of Yarrowia lipolytica lipase. Journal of Molecular Catalysis B: Enzymatic, 39(1–4), 117–121.
Oliveira, D., Feihrmann, A. C., Rubira, A. F., Kunita, M. H., Dariva, C., & Oliveira, J. V. (2006b). Assessment of two immobilized lipases activity treated in compressed fluids. The Journal of Supercritical Fluids, 38, 127–133.
Oliveira, J. V., & Oliveira, D. (2000). Kinetics of enzymatic alcoholysis of palm kernel oil in SC-CO2. Industrial Engineering and Chemical Research, 39, 4450–4455.
Primo, M. S., Ceni, G. C., Marcon, N. S., Antunes, O. A. C., Oliveira, D., Oliveira, J. V., & Dariva, C. (2007). Effects of compressed carbon dioxide treatment on the specificity of oxidase enzymatic complexes from mate tea leaves. Journal of Supercritical Fluids, 43, 283–290.
Steinberger, D. J., Gamse, T., & Maar, R. (1999). Enzyme inactivation and pre-purification effects of supercritical carbon dioxide (SC-CO2). In A. Bertucco (Ed.), Proceedings of the 5th conference on supercritical fluids and their applications (pp. 339–341). Italy: Verona.
Taniguchi, M., Kamihira, M., & Kobayashi, T. (1987). Effect of treatment with supercritical carbon dioxide on enzymatic activity. Agricultural and Biological Chemistry, 51, 593–597.
Tedjo, W., Eshtiaghi, M. N., & Knorr, D. (2000). Impact of supercritical carbon dioxide and high pressure on lipoxygenase and peroxidase activity. Journal of Food Science, 65, 1284–1289.
Acknowledgments
The authors thank CNPq and PROCAD/CAPES for the financial support of this work and scholarships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franken, L.P.G., Marcon, N.S., Treichel, H. et al. Effect of Treatment with Compressed Propane on Lipases Hydrolytic Activity. Food Bioprocess Technol 3, 511–520 (2010). https://doi.org/10.1007/s11947-008-0087-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0087-5