Abstract
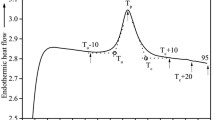
The thermal, dynamic, and structural properties of wheat starch–water systems with different levels of water content (11, 35, 40, 42, 45, and 50%, wet basis) were investigated. 1H time domain nuclear magnetic resonance (TD-NMR) spectroscopy was used to interpret and quantify the water transfer and starch transformations in terms of water uptake, granule swelling, amylose leaching, and melting of starch polymers in relation to the different levels of water content. Complementary differential scanning calorimetry (DSC) experiments were performed to study the effects of water content on the degree of starch gelatinization. In particular, this twofold approach was applied to the first endotherm to study the mechanisms of gelatinization with a common heating range both in NMR and DSC. It was shown that the trend of the enthalpy changes in the first phase transition in starch–water (SW) mixtures was strongly correlated with the loss of solid content measured by NMR in the corresponding temperature range (55–70 °C). Based on the evolution of the relative amplitudes of T 2, structural transformations of starch were shown to occur in both crystalline and amorphous regions within SW samples, supporting the fact that the amorphous phase of starch also plays a significant role in the phase transition of granules during gelatinization. This dynamic and hydrothermal approach provided the first NMR-based interpretation of the first endotherm measured by DSC.







Similar content being viewed by others
References
Ambigaipalan, P., Hoover, R., Donner, E., & Liu, Q. (2014). Starch chain interactions within the amorphous and crystalline domains of pulse starches during heat-moisture treatment at different temperatures and their impact on physicochemical properties. Food Chemistry, 143, 175–184.
Assifaoui, A., Champion, D., Chiotelli, E., & Verel, A. (2006). Rheological behaviour of biscuit dough in relation to water mobility. International Journal of Food Science & Technology, 41(s2), 124–128.
Atwell, W. A. (2001). Composition of commercial flour. Wheat Flour, 27–45.
BeMiller, J. N., & Whistler, R. L. (1996). Carbohydrates. In O. R. Fennema et al. (Eds.), Food chemistry (3rd ed., pp. 191–204). New York: Taylor and Francis Group.
Biliaderis, C. (1992). Structures and phase transitions of starch in food systems. Food Technology, 46(6), 98–109.
Biliaderis, C., Maurice, T., & Vose, J. (1980). Starch gelatinization phenomena studied by differential scanning calorimetry. Journal of Food Science, 45(6), 1669–1674.
Bogracheva, T. Y., Wang, Y., & Hedley, C. (2001). The effect of water content on the ordered/disordered structures in starches. Biopolymers, 58(3), 247–259.
Bosmans, G. M., Lagrain, B., Deleu, L. J., Fierens, E., Hills, B. P., & Delcour, J. A. (2012). Assignments of proton populations in dough and bread using NMR relaxometry of starch, gluten, and flour model systems. Journal of Agricultural and Food Chemistry, 60(21), 5461–5470.
Bosmans, G. M., Pareyt, B., & Delcour, J. A. (2016). Non-additive response of blends of rice and potato starch during heating at intermediate water contents: a differential scanning calorimetry and proton nuclear magnetic resonance study. Food Chemistry, 192, 586–595.
Chinachoti, P., Kimshin, M. S., Mari, F., & Lo, L. (1991). Gelatinization of wheat-starch in the presence of sucrose and sodium-chloride - correlation between gelatinization temperature and water mobility as determined by oxygen-17 nuclear-magnetic-resonance. Cereal Chemistry, 68(3), 245–248.
Chiotelli, E., Pilosio, G., & Le Meste, M. (2002). Effect of sodium chloride on the gelatinization of starch: a multimeasurement study. Biopolymers, 63(1), 41–58.
Choi, S.-G., & Kerr, W. L. (2003). 1H NMR studies of molecular mobility in wheat starch. Food Research International, 36(4), 341–348.
Considine, D. M., & Considine, G. D. (1982). Bread and bakery products. In Foods and food production encyclopedia (pp. 282-295). New York: Van Nostrand Reinhold Co.
Cooke, D., & Gidley, M. J. (1992). Loss of crystalline and molecular order during starch gelatinisation: origin of the enthalpic transition. Carbohydrate Research, 227, 103–112.
Da Silva, C. M., Ciacco, C., Barberis, G., Solano, W., & Rettori, C. (1996). Starch gelatinization measured by pulsed nuclear magnetic resonance. Cereal Chemistry, 73(3), 297–301.
Donald, A. (2004). Understanding starch structure and functionality. In A.-C. Eliasson (Ed.),Starch in food: structure, function and applications (pp. 156–184). Boca Raton: Woodhead Publishing Limited.
Donovan, J. (1979). Phase transitions of the starch–water system. Biopolymers, 18(2), 263–275.
Donovan, J., & Mapes, C. (1980). Multiple phase transitions of starches and Nägeli amylodextrins. Starch-Stärke, 32(6), 190–193.
Donovan, J., Lorenz, K., & Kulp, K. (1983). Differential scanning calorimetry of heat-moisture treated wheat and potato starches. Cereal Chemistry, 60(5), 381-387.
Eliasson, A.-C. (1980). Effect of water content on the gelatinization of wheat starch. Starch-Stärke, 32(8), 270–272.
Eliasson, A.-C., & Larsson, K. (1993). Cereals in breadmaking: a molecular colloidal approach. New York: Marcel Dekker.
Evans, I., & Haisman, D. (1982). The effect of solutes on the gelatinization temperature range of potato starch. Starch-Stärke, 34(7), 224–231.
Fredriksson, H., Silverio, J., Andersson, R., Eliasson, A.-C., & Åman, P. (1998). The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydrate Polymers, 35(3), 119–134.
French, D. (1972). Fine structure of starch and its relationship to the organization of starch granules. 澱粉科学, 19(1), 8–25.
French, D. (1984). Organization of starch granules. Starch: chemistry and technology, 2, 183–247.
Garcia, V., Colonna, P., Lourdin, D., Buleon, A., Bizot, H., & Ollivon, M. (1996). Thermal transitions of cassava starch at intermediate water contents. Journal of Thermal Analysis and Calorimetry, 47(5), 1213–1228.
Godet, M., Bizot, H., & Buléon, A. (1995). Crystallization of amylose—fatty acid complexes prepared with different amylose chain lengths. Carbohydrate Polymers, 27(1), 47–52.
Gonera, A., & Cornillon, P. (2002). Gelatinization of starch/gum/sugar systems studied by using DSC, NMR, and CSLM. Starch-Stärke, 54(11), 508–516.
Greenwood, C. (1979). Observations on the structure of the starch granule. In J. M. V. Blanshard and J. R. Mitchell (Ed.), Polysaccharides in Food (pp. 129-138). London: Butterworths.
Hermansson, A.-M., & Svegmark, K. (1996). Developments in the understanding of starch functionality. Trends in Food Science & Technology, 7(11), 345–353.
Hills, B., Godward, J., Manning, C., Biechlin, J., & Wright, K. (1998). Microstructural characterization of starch systems by NMR relaxation and Q-space microscopy. Magnetic Resonance Imaging, 16(5), 557–564.
Hizukuri, S. (1986). Polymodal distribution of the chain lengths of amylopectins, and its significance. Carbohydrate Research, 147(2), 342–347.
Hoseney, R. C. (1994). Soft wheat products. In Principles of cereal science and technology, (pp. 275-306). St. Paul: American Association of Cereal Chemists.
Jankowski, T., & Rha, C. (1986). Differential scanning calorimetry study of the wheat grain cooking process. Starch-Stärke, 38(2), 45–48.
Jenkins, P. J., & Donald, A. M. (1998). Gelatinisation of starch: a combined SAXS/WAXS/DSC and SANS study. Carbohydrate Research, 308(1), 133–147.
Karapantsios, T., Sakonidou, E., & Raphaelides, S. (2002). Water dispersion kinetics during starch gelatinization. Carbohydrate Polymers, 49(4), 479–490.
Kugimiya, M., Donovan, J., & Wong, R. (1980). Phase transitions of amylose-lipid complexes in starches: a calorimetric study. Starch-Stärke, 32(8), 265–270.
Le Bail, P., Bizot, H., & Buléon, A. (1993). ‘B’to ‘A’type phase transition in short amylose chains. Carbohydrate Polymers, 21(2), 99–104.
Le Botlan, D., Rugraff, Y., Martin, C., & Colonna, P. (1998). Quantitative determination of bound water in wheat starch by time domain NMR spectroscopy. Carbohydrate Research, 308(1), 29–36.
Le Grand, F., Cambert, M., & Mariette, F. (2007). NMR signal analysis to characterize solid, aqueous, and lipid phases in baked cakes. Journal of Agricultural and Food Chemistry, 55(26), 10947–10952.
Lelievre, J. (1974). Starch gelatinization. Journal of Applied Polymer Science, 18(1), 293–296.
Lelievre, J. (1976). Theory of gelatinization in a starch-water-solute system. Polymer, 17(10), 854–858.
Lund, D., & Wirakartakusumah, M. (1984). A model for starch gelatinization phenomena. Engineering and Food, 1, 425–432.
Maache-Rezzoug, Z., Zarguili, I., Loisel, C., Queveau, D., & Buleon, A. (2008). Structural modifications and thermal transitions of standard maize starch after DIC hydrothermal treatment. Carbohydrate Polymers, 74(4), 802–812.
MacArthur, L., & D’appolonia, B. (1979). Comparison of oat and wheat carbohydrates. II. Starch. Cereal Chemistry, 56, 458–461.
Mariette, F., Guillement, J.P., Tellier, C., & Marchal, P. (1996). Continuous relaxation time distribution decomposition by MEM, Chapter 10.
Marquardt, D. W. (1963). An algorithm for least-squares estimation of nonlinear parameters. Journal of the Society for Industrial & Applied Mathematics, 11(2), 431–441.
Medcalf, D., & Gilles, K. (1965). Wheat starches. I. Comparison of physicochemical properties. Cereal Chemistry, 42(558), I965.
Meiboom, S., & Gill, D. (1958). Modified spin-echo method for measuring nuclear relaxation times. Review of Scientific Instruments, 29(8), 688–691.
Morales-Sanchez, E., Figueroa, J., & Gaytan-Martínez, M. (2009). Wet method for measuring starch gelatinization temperature using electrical conductivity. Journal of Food Science, 74(7), E382–E385.
Pojić, M., Musse, M., Rondeau, C., Hadnađev, M., Grenier, D., Mariette, F., et al. (2016). Overall and local bread expansion, mechanical properties, and molecular structure during bread baking: effect of emulsifying starches. Food and Bioprocess Technology, 1–19.
Ratnayake, W. S., & Jackson, D. S. (2008). Starch gelatinization. Advances in Food and Nutrition Research, 55, 221–268.
Rondeau-Mouro, C., Cambert, M., Kovrlija, R., Musse, M., Lucas, T., & Mariette, F. (2015). Temperature-associated proton dynamics in wheat starch-based model systems and wheat flour dough evaluated by NMR. Food and Bioprocess Technology, 8, 777–790.
Sasaki, T., & Matsuki, J. (1998). Effect of wheat starch structure on swelling power. Cereal Chemistry, 75(4), 525–529.
Schirmer, M., Jekle, M., & Becker, T. (2015). Starch gelatinization and its complexity for analysis. Starch-Stärke, 67(1–2), 30–41.
Shi, Y.-C., Capitani, T., Trzasko, P., & Jeffcoat, R. (1998). Molecular structure of a low-amylopectin starch and other high-amylose maize starches. Journal of Cereal Science, 27(3), 289–299.
Shiotsubo, T., & Takahashi, K. (1984). Differential thermal analysis of potato starch gelatinization. Agricultural and Biological Chemistry, 48(1), 9–17.
Shogren, R. (1992). Effect of moisture content on the melting and subsequent physical aging of cornstarch. Carbohydrate Polymers, 19(2), 83–90.
Singh, N., Singh, J., Kaur, L., Singh Sodhi, N., & Singh Gill, B. (2003). Morphological, thermal and rheological properties of starches from different botanical sources. Food Chemistry, 81(2), 219–231.
Slade, L., & Levine, H. (1988). Non-equilibrium melting of native granular starch: part I. Temperature location of the glass transition associated with gelatinization of A-type cereal starches. Carbohydrate Polymers, 8(3), 183–208.
Soulaka, A. B., & Morrison, W. R. (1985). The amylose and lipid contents, dimensions, and gelatinisation characteristics of some wheat starches and their A-and B-granule fractions. Journal of the Science of Food and Agriculture, 36(8), 709–718.
Spigno, G., & De Faveri, D. M. (2004). Gelatinization kinetics of rice starch studied by non-isothermal calorimetric technique: influence of extraction method, water concentration and heating rate. Journal of Food Engineering, 62(4), 337–344.
Svensson, E., & Eliasson, A.-C. (1995). Crystalline changes in native wheat and potato starches at intermediate water levels during gelatinization. Carbohydrate Polymers, 26(3), 171–176.
Tananuwong, K., & Reid, D. S. (2004). DSC and NMR relaxation studies of starch–water interactions during gelatinization. Carbohydrate Polymers, 58(3), 345–358.
Tang, H.-R., Godward, J., & Hills, B. (2000). The distribution of water in native starch granules—a multinuclear NMR study. Carbohydrate Polymers, 43(4), 375–387.
Tang, H.-R., Brun, A., & Hills, B. (2001). A proton NMR relaxation study of the gelatinisation and acid hydrolysis of native potato starch. Carbohydrate Polymers, 46(1), 7–18.
Tester, R., & Sommerville, M. (2001). Swelling and enzymatic hydrolysis of starch in low water systems. Journal of Cereal Science, 33(2), 193–203.
Tester, R., Debon, S., & Sommerville, M. (2000). Annealing of maize starch. Carbohydrate Polymers, 42(3), 287–299.
Waigh, T. A., Gidley, M. J., Komanshek, B. U., & Donald, A. M. (2000). The phase transformations in starch during gelatinisation: a liquid crystalline approach. Carbohydrate Research, 328(2), 165–176.
Wild, D., & Blanshard, J. (1986). The relationship of the crystal structure of amylose polymorphs to the structure of the starch granule. Carbohydrate Polymers, 6(2), 121–143.
Wong, R., & Lelievre, J. (1982). Comparison of the crystallinities of wheat starches with different swelling capacities. Starch-Stärke, 34(5), 159–161.
Wootton, M., & Bamunuarachchi, A. (1979). Application of differential scanning calorimetry to starch gelatinization. I. Commercial native and modified starches. Starch-Stärke, 31(6), 201–204.
Xie, F., Halley, P. J., & Avérous, L. (2012). Rheology to understand and optimize processability, structures and properties of starch polymeric materials. Progress in Polymer Science, 37(4), 595–623.
Acknowledgements
This work was performed using the NMR facilities of the PRISM Research Platform (Rennes, France). The authors thank the Regional Council of Brittany for their financial support. We would also like to thank Denis Lourdin and Marion De Carvalho (INRA Nantes, France) for carrying out the DSC analysis of the starch samples.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Kovrlija, R., Rondeau-Mouro, C. Hydrothermal Changes of Starch Monitored by Combined NMR and DSC Methods. Food Bioprocess Technol 10, 445–461 (2017). https://doi.org/10.1007/s11947-016-1832-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-016-1832-9