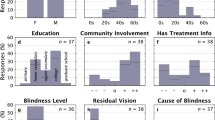
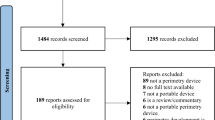
Abstract
With the development of visual prostheses research from the engineering phase to clinical trials, volunteer recruitment for the early visual prosthesis trials needs to be carefully considered. In this article, we mainly discuss several issues related to volunteer recruitment that had posed serious challenges to the visual prosthesis trials, such as low rates of participants, high expectations and underlying motivations to participate in the visual prosthesis trials as well as the importance of informed consent. When recruiting volunteers for visual prosthesis implants, it is critical that the visual prosthesis researchers should not only take into account the patient’s expectations and motivations, but also make the patients fully aware of the possible benefits and risks involved with their participation, and help patients establish realistic expectations for the early phase of visual prosthesis implantation. Based on these considerations to the challenges, eligible volunteers may be recruited in the preliminary stages of visual prosthesis trials.
Similar content being viewed by others
References
Berson, E. L. (1996). Retinitis Pigmentosa: unfolding its mystery. Proceedings of the National academy of Sciences of the United States of America, 93(10), 4526–4528.
Brindley, G. S., & Lewin, W. S. (1968). The sensations produced by electrical stimulation of the visual cortex. The Journal of Physiology, 196(2), 479–493.
Chow, A. Y., & Chow, V. Y. (1997). Subretinal electrical stimulation of the rabbit retina. Neuroscience Letters, 225(1), 13–16.
Chow, A. Y., Chow, V. Y., Packo, K. H., et al. (2004). The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Archives of Ophthalmology, 122, 460–469.
Cohen, E. D. (2007). Safety and effectiveness considerations for clinical studies of visual prosthetic devices. Journal of Neural Engineering, 4(1), S124–S129.
Dagnelie, G. (2008). Psychophysical evaluation for visual prosthesis. Annual Review of Biomedical Engineering, 10, 339–368.
Delbeke, J., Pins, D., Michaux, G., et al. (2001). Electrical stimulation of anterior visual pathways in retinitis pigmentosa. Investigative Ophthalmology & Visual Science, 42(1), 291–297.
Delbeke, J., Wanet-Defalque, M. C., Gerard, B., et al. (2002). The microsystems based visual prosthesis for optic nerve stimulation. Artificial Organs, 26, 232–234.
Dobelle, W. H., Mladejovsky, M. G., & Girvin, J. P. (1974). Artificial vision for the blind: electrical stimulation of visual cortex offers hope for a functional prosthesis. Science, 183(123), 440–444.
Eckmiller, R. (1997). Learning retina implants with epiretinal contacts. Ophthalmic Research, 29(5), 281–289.
Edwards, S. J. L., Lilford, R. J., Thornton, J., & Hewison, J. (1998). Informed consent for clinical trials: in search of the “best” method. Social Science and Medicine, 47(11), 1825–1840.
Emanuel, E. J., Wendler, D., & Grady, C. (2000). What makes clinical research ethical? JAMA, 283(20), 2701–2711.
Humayun, M. S., de Juan, E., Jr., Dagnelie, G., Greenberg, R. J., Propst, R. H., & Phillips, D. H. (1996). Visual perception elicited by electrical stimulation of retina in blind humans. Archives of Ophthalmology, 114(1), 40–46.
Humayun, M. S., de Juan, E., Jr., Weiland, J. D., Dagnelie, G., Katona, S., Greenberg, R. J., et al. (1999). Pattern electrical stimulation of the human retina. Vision Research, 39, 2569–2576.
Humayun, M. S., Weiland, J. D., Fujii, G. Y., et al. (2003). Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Research, 43(24), 2573–2581.
Joffe, L. (1996). The medical and surgical management of ARMD. International Ophthalmology Clinics, 36(2), 99–116.
Levine, R. J. (1988). Ethics and regulation of clinical research (pp. 37–93). New Haven (CT): Yale University Press.
Lidz, C. W., Appelbaum, P. S., Grisso, T., & Renaud, M. (2004). Therapeutic misconception and the appreciation of risks in clinical trials. Social Science Medicine, 58(9), 1689–1697.
Marco, C. A. (2008). Impact of detailed informed consent on research subjects’ participation: a prospective randomized trial. The Journal of Emergency Medicine, 34(3), 269–275.
Merabet, L. B., Rizzo, J. F., I. I. I., Pascual-Leone, A., & Fernandez, E. (2007). ‘Who is the ideal candidate?’: Decisions and issues relating to visual neuroprosthesis development, patient testing and neuroplasticity. Journal of Neural Engineering, 4(1), S130–S135.
Meslin, E. M., Sutherland, H. J., Lavery, J. V., & Till, J. E. (1995). Principlism and the ethical appraisal of clinical trials. Bioethics, 9, 399–418.
National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. (1979). The Belmont report: Ethical principals and guidelines for the protection of human subjects of research. OPRR [Office for Protection from Research Risks] Reports, pp. 1–8.
Normann, R. A., Maynard, E. M., Guillory, K. S., & Warren, D. J. (1996). Cortical implants for the blind. IEEE Spectrum, 112, 54–59.
Richard, C., & Leslie, W. (2006). Artificial vision technology: An early step towards an ethics of cybernetic repair and augmentation. Columbia University Journal of Bioethics, Fall, 59–64.
Rizzo, J., & Wyatt, J. (1997). Prospects for a visual prosthesis. Neuroscientist, 3, 251–262.
Saha, P., & Saha, S. (1986). Ethical responsibilities of the clinical engineer. Journal of Clinical Engineering, 11(1), 17–25.
Schmidt, E. M., Bak, M. J., Hambrecht, F. T., Kufta, C. V., O’Rourke, D. K., & Vallabhanath, P. (1996). Feasibility of a visual prosthesis for the blind based on intracortical microstimulation of the visual cortex. Brain, 119, 507–522.
Thompson, R. W., Barnett, G. D., Humayun, M. S., & Dagnelie, G. (2003). Facial recognition using stimulated prostheric pixelized vision. Investigative Ophthalmology & Visual Science, 44, 5035–5042.
Veraart, C., Duret, F., Brelen, M., et al. (2004). Vision rehabilitation in the case of blindness. Expert Review of Medical Devices, 1, 139–153.
Veraart, C., Raftopoulos, C., Mortimer, J. T., Delbeke, J., Pins, D., Michaux, G., et al. (1998). Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Research, 813(1), 181–186.
Veraart, C., Wanet-Defalque, M. C., Gérard, B., Vanlierde, A., & Delbeke, J. (2003). Pattern recognition with the optic nerve visual prosthesis. Artificial Organs, 27(11), 996–1004.
Weijer, C. (1996). Evolving ethical issues in selection of subjects for clinical research. Cambridge Quarterly of Healthcare Ethics, 5, 334–345.
Yanai, D., Lakhanpal, R. R., Weiland, J. D., et al. (2003). The value of preoperative tests in the selection of blind patients for a permanent microelectronic implant. Transactions of the American Ophthalmological Society, 101, 223–230.
Yu, X., & Qiushi, R. (2010). Preoperative candidate evaluations for retinal prosthesis trials. International Journal of Artificial Organs, 33, 844–850.
Yu, X., Xiujun, P., & Qiushi, R. (2012). Retinitis pigmentosa patients’ attitudes toward participation in retinal prosthesis trials. Contemporary Clinical Trials, 33, 628–632.
Zrenner, E., Miliczek, K. D., Gabel, V. P., Graf, H. G., Guenther, E., Haemmerle, H., et al. (1997). The development of subretinal microphotodiodes for replacement of degenerated photoreceptors. Ophthalmic Research, 29(5), 269–280.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, Y., Ren, Q. Ethical Considerations for Volunteer Recruitment of Visual Prosthesis Trials. Sci Eng Ethics 19, 1099–1106 (2013). https://doi.org/10.1007/s11948-012-9375-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11948-012-9375-6