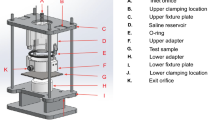
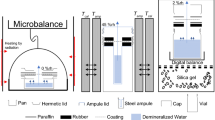
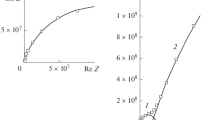
Abstract
Water transport kinetics in the coatings were markedly enhanced and the coating impedances decreased exponentially as temperature increased. The effect of temperature on the coating impedance was attributed to the change in the defect area fraction caused by the thermal expansion of the polymers. The temperature dependence of coating impedance was reversible in a non-aqueous environment but was irreversible in an aqueous environment. This is attributed to the plasticization effect of water on the polymer chains. The effect of glass transition on coating impedances during a short period of exposure to heat source was insignificant.





















Similar content being viewed by others
References
McKenna, GB, “Glass Formation and Glassy Behavior.” In: Allen, G (ed.) Comprehensive Polymer Science and Supplements, pp. 311–362. Pergamon, Amsterdam, 1989
Sperling, LH, Introduction to Physical Polymer Science. Hoboken, Wiley, 2006
Guermazi, V, et al., “Aging Effect on Thermal, Mechanical and Tribological Behaviour of Polymeric Coatings Used for Pipeline Application.” J. Mater. Process. Technol., 203 (1–3) 404–410 (2008)
Guermazi, N, Elleuch, K, Ayedi, HF, “The Effect of Time and Aging Temperature on Structural and Mechanical Properties of Pipeline Coating.” Mater. Des., 30 (6) 2006–2010 (2009)
ASTM, Standard Practice for Operating Salt Spray (Fog) Apparatus, in ASTM B117 - 112003, ASTM International: 100 Barr Harbor Drive, West Conshohocken, PA, USA.
Miszczyk, A, Darowicki, K, “Accelerated Ageing of Organic Coating Systems by Thermal Treatment.” Corros. Sci., 43 (7) 1337–1343 (2001)
Fedrizzi, L, et al., “Assessment of Protective Properties of Organic Coatings by Thermal Cycling.” Progress Org Coat, 48 (2–4) 271–280 (2003)
Bierwagen, GP, et al., “Studies of a New Accelerated Evaluation Method for Coating Corrosion Resistance—Thermal Cycling Testing.” Prog. Org. Coat., 39 (1) 67–78 (2000)
Angeles, ME, Rodriguez, F, Magana, C, “Effect of Heating on the Performance of Anticorrosive Coatings.” Pigm. Resin Technol., 41 (1) 42–48 (2012)
Bierwagen, GP, Tallman, DE, “Choice and Measurement of Crucial Aircraft Coatings System Properties.” Prog. Org. Coat., 41 (4) 201–216 (2001)
Bierwagen, G, et al., “EIS Studies of Coated Metals in Accelerated Exposure.” Prog. Org. Coat., 46 (2) 149–158 (2003)
Li, J, et al., “Thermal Transition Effects and Electrochemical Properties in Organic Coatings: Part 1—Initial Studies on Corrosion Protective Organic Coatings.” Corrosion, 54 (10) 763–771 (1998)
Ochs, H, Vogelsang, J, “Effect of Temperature Cycles on Impedance Spectra of Barrier Coatings Under Immersion Conditions.” Electrochim. Acta, 49 (17–18) 2973–2980 (2004)
Duval, S, et al., “Characterisation of Organic Coatings in Sour Media and Influence of Polymer Structure on Corrosion Performance.” Prog. Org. Coat., 39 (1) 15–22 (2000)
Haruyama, S, Asari, M, Tsuru, T, “Impedance Characteristics during Degradation of Coated Steel.” Corrosion Protection by Organic Coatings. The Electrochemical Society, Pennington, 1987
Scully, JR, “Electrochemical Impedance of Organic-Coated Steel—Correlation of Impedance Parameters with Long-Term Coating Deterioration.” J. Electrochem. Soc., 136 (4) 979–990 (1989)
Kendig, M, Scully, J, “Basic Aspects of Electrochemical Impedance Application for the Life Prediction of Organic Coatings on Metals.” Corrosion, 46 (1) 22–29 (1990)
Mansfeld, F, “Electrochemical Impedance Spectroscopy (Eis) as a New Tool for Investigating Methods of Corrosion Protection.” Electrochim. Acta, 35 (10) 1533–1544 (1990)
Hack, HP, Scully, JR, “Defect Area Determination of Organic Coated Steels in Seawater Using the Breakpoint Frequency Method.” J. Electrochem. Soc., 138 (1) 33–40 (1991)
Murray, JN, Hack, HP, “Long-Term Testing of Epoxy-Coated Steel in ASTM Seawater Using Electrochemical Impedance Spectroscopy.” Corrosion, 47 (6) 480–489 (1991)
Mansfeld, F, White, RE, “Comparing Electrochemical Impedance Spectroscopy Methods for Estimating the Degree of Delamination of Organic Coatings on Steel.” J. Electrochem. Soc., 140 (6) 1825 (1993)
van Westing, EPM, Ferrari, GM, De Wit, JHW, “The Determination of Coating Performance Using Electrochemical Impedance Spectroscopy.” Electrochim. Acta, 39 (7) 899–910 (1994)
Brasher, DM, Kingsbury, AH, “Electrical Measurements in the Study of Immersed Paint Coatings On Metal. I. Comparison Between Capacitance and Gravimetric Methods of Estimating Water-Uptake.” J. Appl. Chem., 4 (2) 62–72 (1954)
Castela, AS, Simões, AM, “Assessment of Water Uptake in Coil Coatings by Capacitance Measurements.” Prog. Org. Coat., 46 (1) 55–61 (2003)
Mansfeld, F, Tsai, CH, “Discussion on the Relationship of Break-Point Frequencies to Delamination.” Corrosion, 47 (12) 964–965 (1991)
Hack, H, “Defect Area Determination of Organic-Coated Steels in Seawater Using the Breakpoint Frequency Method—Reply.” J. Electrochem. Soc., 139 (2) 639–640 (1992)
Mansfeld, F, “Use of Electrochemical Impedance Spectroscopy for the Study of Corrosion Protection by Polymer Coatings—Reply.” J. Appl. Electrochem., 25 (12) 1145 (1995)
Scully, JR, Hensley, ST, “Lifetime Prediction for Organic Coatings on Steel and a Magnesium Alloy Using Electrochemical Impedance Methods.” Corrosion, 50 (9) 705–716 (1994)
Hinderliter, BR, et al., “Thermal Cycling of Epoxy Coatings Using Room Temperature Ionic Liquids.” J. Electrochem. Soc., 155 (3) C93–C100 (2008)
Upadhyay, V, et al., “Preliminary Investigation of the Impact of Polymer Composition on Electrochemical Properties of Coatings as Determined by Electrochemical Impedance Spectroscopy.” J. Coat. Technol. Res., 10 (6) 865–878 (2013)
Simões, AM, Tallman, DE, Bierwagen, GP, “Use of Ionic Liquids for the Electrochemical Characterization of Water Transport in Organic Coatings.” Electrochem. Solid-State Lett., 8 (10) B60–B63 (2005)
International, A., ASTM E2602 - 09 Standard Test Method for the Assignment of the Glass Transition Temperature by Modulated Temperature Differential Scanning Calorimetry, 2009, ASTM International: West Conshohocken, PA.
Thomas, NL, “The Barrier Properties of Paint Coatings.” Prog. Org. Coat., 19 (2) 101–121 (1991)
Crank, J, The Mathematics of Diffusion, 2nd ed. Clarendon Press, Oxford, 1975
Grundmeier, G, Schmidt, W, Stratmann, M, “Corrosion Protection by Organic Coatings: Electrochemical Mechanism and Novel Methods of Investigation.” Electrochim. Acta, 45 (15–16) 2515–2533 (2000)
Granata, RD, Kovaleski, KJ, “Evaluation of High-Performance Protective Coatings by Electrochemical Impedance and Chronoamperometry.” Electrochem. Impedence, 1188 450–462 (1993)
Tait, WS, et al., “Analyzing and Interpreting Electrochemical Impedance Spectroscopy Data from Internally Coated Steel Aerosol Containers.” Electrochem. Impedence, 1188 428–437 (1993)
Oliveira, CG, Ferreira, MGS, “Ranking High-Quality Paint Systems Using EIS. Part 1: Intact Coatings.” Corros. Sci., 45 (1) 123–138 (2003)
Oliveira, CG, Ferreira, MGS, “Ranking High-Quality Paint Systems Using EIS. Part II: Defective Coatings.” Corros. Sci., 45 (1) 139–147 (2003)
Choi, S, Douglas, EP, “Complex Hygrothermal Effects on the Glass Transition of an Epoxy-Amine Thermoset.” ACS Appl. Mater. Interfaces, 2 (3) 934–941 (2010)
Taylor, LS, Langkilde, FW, Zografi, G, “Fourier Transform Raman Spectroscopic Study of the Interaction of Water Vapor with Amorphous Polymers.” J. Pharm. Sci., 90 (7) 888–901 (2001)
Myllytie, P, et al., “Viscoelasticity and Water Plasticization of Polymer-Cellulose Composite Films and Paper Sheets.” Cellulose, 17 (2) 375–385 (2010)
Kelley, FN, Bueche, F, “Viscosity and Glass Temperature Relations for Polymer-Diluent systems.” J. Polym. Sci., 50 (154) 549–556 (1961)
Zhou, J, Lucas, JP, “Hygrothermal Effects of Epoxy Resin. Part I: The Nature of Water in Epoxy.” Polymer, 40 (20) 5505–5512 (1999)
Zhou, J, Lucas, JP, “Hygrothermal Effects of Epoxy Resin. Part II: Variations of Glass Transition Temperature.” Polymer, 40 (20) 5513–5522 (1999)
Philippe, LVS, et al., “Validation of Electrochemical Impedance Measurements for Water Sorption into Epoxy Coatings Using Gravimetry and Infra-red Spectroscopy.” Corros. Sci., 50 (3) 887–896 (2008)
Cao-Paz, A, et al., “Ingress of Water into Organic Coatings: Real-Time Monitoring of the Capacitance and Increase in Mass.” Prog. Org. Coat., 69 (2) 150–157 (2010)
Ayres, E, Vasconcelos, WL, Oréfice, RL, “Attachment of Inorganic Moieties onto Aliphatic Polyurethanes.” Mater. Res., 10 119–125 (2007)
Gómez-Sánchez, E, et al., “ATR-FTIR Spectroscopy for the Characterisation of Magnetic Tape Materials. E-Preservation.” Sci. Sports, 8 2–9 (2010)
Es-sebbar, E-T, Benilan, Y, Farooq, A, “Temperature-Dependent Absorption Cross-Section Measurements of 1-Butene (1-C4H8) in VUV and IR.” J. Quant. Spectrosc. Radiat. Transf., 115 1–12 (2013)
Bešter-Rogač, M, Neueder, R, Barthel, J, “Conductivity of Sodium Chloride in Water + 1,4-Dioxane Mixtures from 5 to 35°C II. Concentrated Solution.” J. Solut. Chem., 29 (1) 51–61 (2000)
Acknowledgments
The authors would like to thank Specialty Polymer Coatings Inc. and Natural Sciences and Engineering Research Council of Canada (NSERC) for their financial and technical support of this project. The discussion with Dr. Farzad Mohammadi in the Corrosion Group at UBC is highly acknowledged. The authors are also grateful for the help in DSC and FTIR experiments from Dr. Christophe Mobuchon and Mr. Arjun Sethi at the Composite Research Network (CRN) at UBC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ha, H.M., Alfantazi, A. On the role of water, temperature, and glass transition in the corrosion protection behavior of epoxy coatings for underground pipelines. J Coat Technol Res 12, 1095–1110 (2015). https://doi.org/10.1007/s11998-015-9705-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-015-9705-0