Abstract
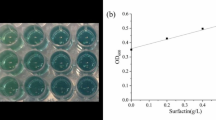
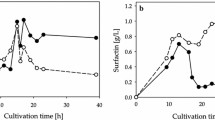
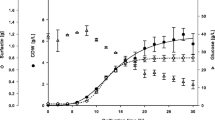
Bacillus subtilis BS5 is a soil isolate that produces promising yield of surfactin biosurfactant in mineral salts medium (MSM). It was found that cellular growth and surfactin production in MSM were greatly affected by the environmental fermentation conditions and the medium components (carbon and nitrogen sources and minerals). Optimum environmental conditions for high surfactin production on the shake flask level were found to be a slightly acidic initial pH (6.5–6.8), an incubation temperature of 30°C, a 90% volumetric aeration percentage, and an inoculum size of 2% v/v. For media components, it was found that the optimum carbon source was molasses (160 ml/l), whereas the optimum nitrogen source was NaNO3 (5 g/l) and the optimum trace elements were ZnSO4·7H2O (0.16 g/l), FeCl3·6H2O (0.27 g/l), and MnSO4·H2O (0.017 g/l). A modified MSM (molasses MSM), combining the optimum medium components, was formulated and resulted in threefold increase in surfactin productivity that reached 1.12 g/l. No plasmid could be detected in the tested isolate, revealing that biosurfactant production by B. subtilis isolate BS5 is chromosomally mediated but not plasmid-mediated.

















Similar content being viewed by others
References
Makkar, R. S., & Cameotra, S. S. (1999). Biosurfactant production by microorganisms on unconventional carbon sources. Journal of Surfactants and Detergents, 2, 237–241.
Sanchez, M., Teruel, J. A., Espuny, M. J., Marques, A., Aranda, F. J., Manresa, A., et al. (2006). Modulation of the physical properties of dielaidoylphosphatidylethanolamine membranes by a dirhamnolipid biosurfactant produced by Pseudomonas aeruginosa. Chemistry and Physics of Lipids, 142, 118–127.
Banat, I. M. (1995). Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: a review. Bioresource Technology, 51, 1–12.
Desai, J. D., & Banat, I. M. (1997). Microbial production of surfactants and their commercial potential. Microbiology and Molecular Biology Reviews, 61(1), 47–64.
Nitschke, M., & Pastore, G. M. (2004). Biosurfactant production by Bacillus subtilis using cassava-processing effluent. Applied Biochemistry and Biotechnology, 112(3), 163–172.
Cooper, D. G., Macdonald, T. C. R., Duff, S. J. B., & Kosaric, N. (1981). Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation additions. Applied and Environmental Microbiology, 42(3), 408–412.
Arima, K., Kakinuma, A., & Tamura, G. (1968). Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochemical and Biophysical Research Communications, 31, 488–494.
Vollenbroich, D., Ozel, M., Vater, J., Kamp, R. M., & Pauli, G. (1997). Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus Subtilis. Biologicals, 25, 289–297.
Vollenbroich, D., Pauli, G., Ozel, M., & Vater, J. (1997). Antimycoplasma properties and application in cell culture of surfactin, a lipopeptide antibiotic from Bacillus Subtilis. Applied and Environmental Microbiology, 63(1), 44–49.
Nitschke, M., Ferraz, C., & Pastore, G. M. (2004). Selection of microorganisms for biosurfactant production using agroindustrial wastes. Brazilian Journal of Microbiology, 35, 81–85.
Deleu, M., & Paquot, M. (2004). From renewable vegetables resources to microorganisms: new trends in surfactants. Comptes Rendus Chimie, 7, 641–646.
Hsieh, F.-C., Li, M.-C., Lin, T.-C., & Kao, S.-S. (2004). Rapid detection and characterization of surfactin-producing Bacillus subtilis and closely related species based on PCR. Current Microbiology, 49, 186–191.
Makkar, R. S., & Cameotra, S. S. (1997). Utilization of molasses for biosurfactant production by two Bacillus strains at thermophilic conditions. JAOCS, 74, 887–889.
Mulligan, C. N., & Cooper, D. G. (1985). Pressate from peat dewatering as a substrate for bacterial growth. Applied and Environmental Microbiology, 50(1), 160–162.
Fox, S. L., & Bala, G. A. (2000). Production of surfactant from Bacillus subtilis ATCC 21332 using potato substrates. Bioresource Technology, 75, 235–240.
Manresa, M. A., Bastida, J., Mercade, M. E., Robert, M., de Andres, C., Espuny, M. J., et al. (1991). Kinetic studies on surfactant production by Pseudomonas aeruginosa 44T1. Journal of Industrial Microbiology and Biotechnology, 8(2), 133–136.
Guerra-Santos, L. H., Kappeli, O., & Fiechter, A. (1986). Dependence of Pseudomonas aeruginosa continous culture biosurfactant production on nutritional and environmental factors. Applied Microbiology and Biotechnology, 24(6), 443–448.
Chayabutra, C., Wu, J., & Ju, L.-K. (2001). Rhamnolipid production by Pseudomonas aeruginosa under denitrification: Effects of limiting nutrients and carbon substrates. Biotechnology and Bioengineering, 72(1), 25–33.
Lang, S., & Wullbrandt, D. (1999). Rhamnose lipids—biosynthesis, microbial production and application potential. Applied Microbiology and Biotechnology, 51(1), 22–32.
Wei, Q. F., Mather, R. R., & Fotheringham, A. F. (2005). Oil removal from used sorbents using a biosurfactant. Bioresource Technology, 96, 331–334.
Nitschke, M., Costa, S. G. V. A. O., & Contiero, J. (2005). Rhamnolipid surfactants: An update on the general aspects of these remarkable biomolecules. Biotechnology Progress, 21, 1593–1600.
Abdel-Mawgoud, A. M., Aboulwafa, M. M., & Hassouna, N. A.-H. (2007). Microbial production of surfactants: screening and identification of two promising isolates and their biosurfactants. Egyptian Journal of Biotechnology, 27, in press.
Alfermann, A. W., Dombrowski, K., Petersen, M., Schmauder, H.-P., & Schweizer, M. (1997). Basic scientific techniques for biotechnology—Analytical methods—Growth and cell viability. In H.-P. Schmauder, & M. Schweizer (Eds.) Methods in biotechnology ((pp. 13–14)2nd ed.). London: Taylor & Francis.
Morikawa, M., Hirata, Y., & Imanaka, T. (2000). A study on the structure–function relationship of lipopeptide biosurfactants. Biochimica et Biophysica Acta, 1488, 211–218.
Youssef, N. H., Duncana, K. E., Naglea, D. P., Savagea, K. N., Knappb, R. M., & McInerney, M. J. (2004). Comparison of methods to detect biosurfactant production by diverse microorganisms. Journal of Microbiological Methods, 56, 339–347.
Morikawa, M., Daido, H., Takao, T., Murata, S., Shimonishi, Y., & Imanaka, T. (1993). A new lipopeptide biosurfactant produced by Arthrobacter sp. strain MIS38. Journal of Bacteriology, 175(20), 6459–6466.
Birnboim, H. C., & Doly, J. (1979). A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Research, 7, 1513–1523.
Sambrouk, J., & Russell, D. W. (2001). Molecular cloning: A laboratory manual (3rd ed.). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Gershater, C. J. L. (1999). Inoculum preparation. In M. C. Flickinger, & S. W. Drew (Eds.) Encyclopedia of bioprocess technology: Fermentation, biocatalysis, and bioseparation (pp. 1435–1443). New York: Wiley.
Lim, D. (1998). Nutrition and environmental influence. In E. W. Lester (Ed.) Microbiology: A human perspective(2nd ed.). Boston, MA: McGraw-Hill.
Kim, H.-S., Yoon, B.-D., Lee, C.-H., Suh, H.-H., Oh, H.-M., Katsuragi, T., & Tani, Y. (1997). Production and properties of a lipopeptide biosurfactant from Bacillus subtilis C9. Journal of Fermentation and Bioengineering, 84(1), 41–46.
Smith, J. E. (1996). Bioprocess/fermentation technology. In: Biotechnology (3rd ed., pp. 60–61). UK: Cambridge University Press.
Macdonald, C. R., Cooper, D. G., & Zajic, J. E. (1981). Surface-active lipids from Nocardia erythropolis grown on hydrocarbons. Applied and Environmental Microbiology, 41(1), 117–123.
Banat, I. M. (1993). The isolation of a thermophilic biosurfactant producing Bacillus sp. Biotechnology Letters, 15(6), 591–594.
Horowitz, S., Gilbert, J. N., & Griffin, W. M. (1990). Isolation and characterization of a surfactant produced by Bacillus licheniformis 86. Journal of Industrial Microbiology, 6, 243–248.
Javaheri, M., Jenneman, G. E., Mcinerney, M. J., & Knapp, R. M. (1985). Anaerobic production of a biosurfactant by Bacillus licheniformis JF-2. Applied and Environmental Microbiology, 50(3), 698–700.
Lin, S. C., Carswell, K. S., Sharma, M. M., & Georgiou, G. (1994). Continuous production of the lipopeptide biosurfactant of Bacillus licheniformis JF-2. Applied Microbiology and Biotechnology, 41, 281–285.
Yakimov, M. M., Timmis, K. N., Wray, V., & Fredrickson, H. L. (1995). Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Applied and Environmental Microbiology, 61(5), 1706–1713.
Koch, A. K., Kappeli, O., Fiechter, A., & Reiser, J. (1991). Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. Journal of Bacteriology, 173(13), 4212–4219.
Peypoux, F., Bonmatin, J. M., & Wallach, J. (1999). Recent trends in the biochemistry of surfactin. Applied Microbiology and Biotechnology, 51, 553–563.
Landy, M., Warren, G. H., Roseman, S. B., & Colio, L. G. (1948). Bacillomycin: an antibiotic from Bacillus subtilis active against pathogenic fungi. Proceedings of the Society for Experimental Biology and Medicine, 67, 539–541.
Wei, Y.-H., & Chu, I.-M. (1998). Enhancement of surfactin production in iron-enriched media by Bacillus subtilis ATCC 21332. Enzyme and Microbial Technology, 22, 724–728.
Wei, Y.-H., & Chu, I.-M. (2002). Mn2 improves surfactin production by Bacillus subtilis. Biotechnology Letters, 24, 479–482.
Fleck, L. C., Bicca, F. C., & Ayub, M. A. Z. (2000). Physiological aspects of hydrocarbon emulsification, metal resistance and DNA profile of biodegrading bacteria isolated from oil polluted sites. Biotechnology Letters, 22, 285–289.
Acknowledgements
The authors would like to thank Dr. Khaled Abou-Shanab, a lecturer of Microbiology and Immunology, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt, for kindly providing the standard E. coli DH5α/pUC18 and giving advice on plasmid detection in the tested isolate.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Mawgoud, A.M., Aboulwafa, M.M. & Hassouna, N.AH. Optimization of Surfactin Production by Bacillus subtilis Isolate BS5. Appl Biochem Biotechnol 150, 305–325 (2008). https://doi.org/10.1007/s12010-008-8155-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8155-x