Abstract
Recovering hydrolysis enzymes and/or alternative enzyme addition strategies are two potential mechanisms for reducing the cost during the biochemical conversion of lignocellulosic materials into renewable biofuels and biochemicals. Here, we show that enzymatic hydrolysis of acid-pretreated pine wood with continuous and/or fed-batch enzyme addition improved sugar conversion efficiencies by over sixfold. In addition, specific activity of the hydrolysis enzymes (cellulases, hemicellulases, etc.) increased as a result of continuously washing the residual solids with removal of glucose (avoiding the end product inhibition) and other enzymatic inhibitory compounds (e.g., furfural, hydroxymethyl furfural, organic acids, and phenolics). As part of the continuous hydrolysis, anion exchange resin was tested for its dual application of simultaneous enzyme recovery and removal of potential enzymatic and fermentation inhibitors. Amberlite IRA-96 showed favorable adsorption profiles of inhibitors, especially furfural, hydroxymethyl furfural, and acetic acid with low affinity toward sugars. Affinity of hydrolysis enzymes to adsorb onto the resin allowed for up to 92 % of the enzymatic activity to be recovered using a relatively low-molar NaCl wash solution. Integration of an ion exchange column with enzyme recovery into the proposed fed-batch hydrolysis process can improve the overall biorefinery efficiency and can greatly reduce the production costs of lignocellulosic biorenewable products.

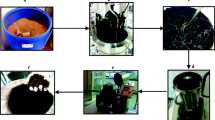
A semicontinuous process for the biochemical production of renewable products using detoxification and fed-batch enzyme addition/recycle can increase enzymatic hydrolysis and fermentation efficiencies. Hydrolysis enzymes, inhibitors, sugars, and water can be separated and utilized as high-value steams within the process









Similar content being viewed by others
References
Kamm, B., & Kamm, M. (2004). Principles of biorefineries. Appl Microbiol Biotechnol, 64(2), 137–145.
Ohara, H. (2003). Biorefinery. Appl Microbiol Biotechnol, 62(5/6), 474–477.
Wilke, T., & Vorlop, K. D. (2004). Industrial conversion of renewable resources as an alternative to conventional chemistry. Appl Microbiol Biotechnol, 66(2), 131–142.
Hamelinck, C. N., van Hooijdonk, G., & Faajj, A. P. C. (2005). Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenergy, 28(4), 384–410.
Mosier, N., Wyman, C., Dale, B. E., Elander, R., Lee, Y. Y., Holtzapple, M., & Ladisch, M. (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol, 96(6), 673–686.
Menon, V., & Rao, M. (2012). Trends in bioconversion of lignocelluloses: biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci, 38(4), 522–550.
Jacquet, N., Vanderghem, C., Blecker, C., Malumba, P., Delvigne, F., & Paquot, M. (2012). Improvement of the cellulose hydrolysis yields and hydrolysate concentration by management of enzymes and substrate input. Cerevisia, 37(3), 82–87.
Laureano-Perez, L., Teymouri, F., Alizadeh, H., & Dale, B. E. (2005). Understanding factors that limit enzymatic hydrolysis of biomass. Appl Biochem Biotechnol, 121–124(1–3), 1081–1099.
Yang, B., & Wyman, C. E. (2007). Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Biorefin, 2(1), 26–40.
Gregg, D. J., Boussaid, A., & Saddler, J. (1998). Techno-economic modeling of a generic wood-to-ethanol process: effect of increased cellulose yields and enzyme recycle. Bioresour Technol, 63(1), 7–12.
Wingren, A., Galbe, M., & Zacchi, G. (2003). Techno-economic evaluation of producing ethanol from softwood: comparison of SSF and SHF and identification of bottlenecks. Biotechnol Prog, 19(4), 1109–1117.
Galbe, M., & Zacchi, G. (2002). A review of the production of ethanol from softwood. Appl Microbiol Biotechnol, 59(6), 618–628.
Klein-Marcuschamer, D., Oleskowicz-Popiel, P., Simmons, B. A., & Blanch, H. W. (2012). The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng, 109(4), 1083–1087.
Berlin, A., Gilkes, A., Kurabi, A., Bura, R., Tu, M. B., & Kilburn, D. (2005). Weak lignin-binding enzymes—a novel approach to improve activity of cellulases for hydrolysis of lignocellulosics. Appl Biochem Biotechnol, 121/124, 163–170.
Gusakov, A. V., Salanovich, T. N., Antonov, A. I., Ustinov, B. B., Okunev, O. N., & Bulingame, R. (2007). Design of highly efficient cellulase mixtures for enzymatic hydrolysis of cellulose. Biotechnol Bioeng, 97(5), 1028–1038.
Zhou, J., Wang, Y. H., Chua, J., Luoa, L. Z., Zhuanga, Y. P., & Zhanga, S. L. (2009). Optimization of cellulase mixture for efficient hydrolysis of steam-exploded corn stover by statistically designed experiments. Bioresour Technol, 100(2), 819–825.
Zhang, Y. H. P., Himmel, M. E., & Mielenz, J. R. (2006). Outlook for cellulase improvement: screening and selection strategies. Biotechnol Adv, 4(3), 201–210.
Muhammad, S. A., Mahjabeen, S., & Waheed, A. (2001). Saccharification of lignocellulosic materials by the cellulases of Bacillus subtilis. Int J Agric Biol, 3(2), 199–202.
Srinorakutar, T., Subkaree, Y., Bamrungchue, N., Pripanapong, P., & Burapatana, V. (2012). Effect of lignocellulosic substrate and commercial cellulase loading on reducing sugar concentration for ethanol production. J Food Sci Eng, 2(3), 149–156.
Girard, D. J., & Converse, A. O. (1993). Recovery of cellulase from lignaceous hydrolysis residue. Appl Biochem Biotechnol, 39/40(1), 521–533.
Lee, D., Yu, A. H. C., Wong, K. K. Y., & Saddler, J. N. (1994). Evaluation of the enzymatic susceptibility of cellulosic substrates using specific hydrolysis rates and enzyme adsorption. Appl Biochem Biotechnol, 45/46(1), 407–415.
Gurram, R. N., Datta, S., Lin, Y. J., Snyder, S. W., & Menkhaus, T. J. (2011). Removal of enzymatic and fermentation inhibitory compounds from biomass slurries for enhanced biorefinery process efficiencies. Bioresour Technol, 102(17), 7850–7859.
Jing, X., Zhang, X., & Ba, J. (2009). Inhibition performance of lignocellulose degradation products on industrial cellulase enzymes during cellulose hydrolysis. Appl Biochem Biotechnol, 159(3), 696–707.
Szengyel, Z., & Zacchi, G. (2000). Effect of acetic acid and furfural on cellulase production of Trichoderma reesei RUT C30. Appl Biochem Biotechnol, 89(1), 31–42.
Phillippidis, G. P., Smith, T. K., & Wyman, C. E. (1992). Study of the enzymatic hydrolysis of cellulose for production of fuel ethanol by the simultaneous saccharification and fermentation process. Biotechnol Bioeng, 41(9), 846–853.
Xiao, Z., Zhang, X., Gregg, D. J., & Saddler, J. N. (2004). Effects of sugar inhibition on cellulases and β-glucosidase during enzymatic hydrolysis of softwood substrates. Appl Biochem Biotechnol, 113/116, 1115–1126.
Lu, Y. P., Yang, B., Gregg, D., Saddler, J. N., & Mansfield, S. D. (2002). Cellulase adsorption and an evaluation of enzyme recycle during hydrolysis of steam-exploded softwood residues. Appl Biochem Biotechnol, 98/100, 641–654.
Meshartree, M., Hogan, C. M., & Saddler, J. N. (1987). Recycle of enzymes and substrate following enzymatic hydrolysis of steam-pretreated aspenwood. Biotechnol Bioeng, 30(4), 558–564.
Deshpande, M. V., & Eriksson, K. E. (1984). Reutilization of enzymes for saccharification of lignocellulosic materials. Enzym Microbiol Technol, 6(8), 338–340.
Ooshima, H., Burn, D. S., & Converse, A. O. (1990). Adsorption of cellulase from Trichoderma reesei on cellulose and lignacious residue in wood pretreated by dilute sulfuric acid with explosive decompression. Biotechnol Bioeng, 36(5), 446–452.
Rao, R., & Radhakrishnan, D. (2008). Dynamics of cellulase activity during composting of municipal solid waste. Electron J Environ Agric Food Chem, 7(9), 3191–3198.
Sinitsyn, A. P., Bungay, M. L., Clesceri, L. S., & Bungay, H. R. (1983). Recovery of enzymes from the insoluble residue of hydrolyzed wood. Appl Biochem Biotechnol, 8(1), 25–29.
Knutsen, J. S., & Davis, R. H. (2004). Cellulase retention and sugar removal by membrane ultrafiltration during lignocellulosic biomass hydrolysis. Appl Biochem Biotechnol, 113/116, 585–599.
Ramos, L. P., Breuil, C., & Saddler, J. N. (1993). The use of enzyme recycling and the influence of sugar accumulation on cellulose hydrolysis by Trichoderma cellulases. Enzym Microbiol Technol, 15(1), 19–25.
Tu, M., Chandra, R. P., & Saddler, J. N. (2007). Evaluating the distribution of cellulases and the recycling of free cellulases during the hydrolysis of lignocellulosic substrates. Biotechnol Prog, 23(2), 398–406.
Qi, B., Luo, J., Chen, G., Chen, X., & Wan, Y. (2012). Application of ultrafiltration and nanofiltration for recycling cellulase and concentrating glucose from enzymatic hydrolysate of steam exploded wheat straw. Bioresour Technol, 104, 466–472.
Ramos, L. P., & Saddler, J. N. (1994). Enzyme recycling during fed-batch hydrolysis of cellulose derived from steam-exploded Eucalyptus viminalis. Appl Biochem Biotechnol, 45/46, 193–207.
Steele, B., Raj, S., Nghiem, J., & Stowers, M. (2005). Enzyme recovery and recycling following hydrolysis of ammonia fiber explosion-treated corn stover. Appl Biochem Biotechnol, 121/124, 901–910.
Tu, M., Chandra, R. P., & Saddler, J. N. (2007). Recycling cellulases during the hydrolysis of steam exploded and ethanol pretreated lodgepole pine. Biotechnol Prog, 23(5), 1130–1137.
Weiss, N., Borjesson, J., Pedersen, L. S., & Meyer, A. S. (2013). Enzymatic lignocelluloses hydrolysis: improved cellulase productivity by insoluble solids recycling. Biotech Biofuels, 6, 5. doi:10.1186/1754-6834-6-5.
Mores, W. D., Knutsen, J. S., & Davis, R. H. (2001). Cellulase recovery via membrane filtration. Appl Biochem Biotechnol, 91/93, 297–309.
Roche, C. M., Dibble, C. J., & Stickel, J. J. (2009). Laboratory-scale method for enzymatic saccharification of lignocellulosic biomass at high-solids loadings. Biotech Biofuels, 2, 28. doi:10.1186/1754-6834-2-28.
Junker, B. (2007). Foam and its mitigation in fermentation system. Biotechnol Prog, 23(4), 767–784.
Hu, C. Y., & Lin, L. P. (2003). Characterization and purification of hydrolytic enzymes in Sinorhizobium fredii CCRC 15769. World J Microbiol Biotechnol, 19(5), 515–522.
Li, Y. H., Ding, M., Wang, J., Xu, G. J., & Zhao, F. (2006). A novel thermoacidophilic endoglucanase, B-EGA, from a new cellulose-degrading bacterium, Bacillus sp. AC-1. Appl Biochem Biotechnol, 70(4), 430–436.
Singh, J., Batra, N., & Sobti, R. C. (2004). Purification and characterization of alkaline cellulose produced by a novel isolate, Bacillus sphaericus JS1. Ind Microbiol Biotechnol, 31(2), 51–56.
Larsson, S., Reimann, A., Nilvebrant, N., & Jönsson, L. (1999). Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl Biochem Biotechnol, 77(1), 91–103.
Ranjan, R., Thust, S., Gounaris, C. E., Woo, M., Floudas, C. A., Keitz, M., Valentas, K. J., Wei, J., & Tsapatsis, M. (2009). Adsorption of fermentation inhibitors from lignocellulosic biomass hydrolyzates for improved ethanol yield and value-added product recovery. Microporous Mesoporous Mater, 122(1/3), 143–148.
Sainio, T., Turku, I., & Heinonen, J. (2011). Adsorptive removal of fermentation inhibitors from concentrated acid hydrolyzates of lignocellulosic biomass. Bioresour Technol, 102(10), 6048–6057.
Carter, B., Gilcrease, P. C., & Menkhaus, T. J. (2011). Removal and recovery of furfural, 5-hydroxymethylfurfural and acetic acid from aqueous solutions using a soluble polyelectrolyte. Biotechnol Bioeng, 108(9), 2046–2052.
Carter, B., Squillace, P., Gilcrease, P. C., & Menkhaus, T. J. (2011). Detoxification of a lignocellulosic biomass slurry by soluble polyelectrolyte adsorption for improved fermentation efficiency. Biotechnol Bioeng, 108(9), 2053–2060.
Burke, D. R., Anderson, J., Gilcrease, P. C., & Menkhaus, T. J. (2011). Enhanced solid–liquid clarification of lignocellulosic slurries using polyelectrolyte flocculating agents. Biomass Bioenergy, 35(1), 391–401.
Zhou, H., Lou, H., Yan, D., Zhu, J. Y., & Qiu, X. (2013). Lignosulfonate to enhance enzymatic saccharification of lignocelluloses: role of molecular weight and substrate lignin. Ind Eng Chem Resour, 52(25), 8464–8470.
Arantes, V., & Saddler, J. N. (2011). Cellulose accessibility limits the effectiveness of minimum cellulose loading on the efficient hydrolysis of pretreated lignocellulosic substrates. Biotech Biofuels, 4, 3. doi:10.1186/1754-6834-4-3.
Gan, Q., Allen, S. J., & Taylor, G. (2003). Kinetic dynamics in heterogeneous enzymatic hydrolysis of cellulose: an overview, an experimental study and mathematical modeling. Process Biochem, 38, 1003–1018.
Lee, Y. Y., Iyer, P., & Torget, R. W. (1999). Dilute-acid hydrolysis of lignocellulosic biomass. Adv Biochem Eng Biotechnol, 65, 93–115.
Tan, L. U. L., Yu, E. K. C., Campbell, N., & Saddler, J. N. (1986). Column cellulose hydrolysis reactor: an efficient cellulose hydrolysis reactor with continuous cellulase recycling. Appl Microbiol Biotechnol, 25(3), 250–255.
Palmqvist, E., Grage, H., Meinander, N. Q., & Hahn-Hagerdal, B. (1999). Main and interaction effects of acetic acid, furfural, and p-hydroxybenzoic acid on growth and ethanol productivity of yeasts. Biotechnol Bioeng, 63(1), 46–55.
Rachma, W., Ria, M., Siti, S., Ririn, M., & Yulian, A. (2010). Effect of furfural, hydroxymethylfurfural and acetic acid on indigenous microbial isolate for bioethanol production. Agric J, 5(2), 105–109.
Klinke, H. B., Thomsen, A. B., & Ahring, B. K. (2004). Inhibition of ethanol producing yeast and bacteria by degradation products produced during pretreatment of biomass. Appl Microbiol Biotechnol, 66(1), 10–26.
Ximenes, E., Kim, Y., Mosier, N. S., Dien, B., & Ladisch, M. R. (2010). Inhibition of cellulases by phenols. Enzym Microb Technol, 46, 170–176.
Kim, Y., Ximenes, E., Mosier, N. S., & Ladisch, M. R. (2011). Soluble inhibitors/deactivators of cellulose enzymes from lignocellulosic biomass. Enzym Microb Technol, 48(4–5), 408–415.
Weil, J. R., Dien, B., Bothast, R., Hendrickson, R., Mosier, N. S., & Ladisch, M. R. (2002). Removal of fermentation inhibitors formed during pretreatment of biomass by polymeric adsorbents. Ind Eng Chem Res, 41(24), 6132–6138.
Luo, X., Gleisner, R., Tian, S., Negron, J., Horn, E., Pan, X. J., & Zhu, J. Y. (2010). Evaluation of mountain beetle-infested lodgepole pine for cellulosic ethanol production by SPORL pretreatment. Ind Eng Chem Res, 49(17), 8258–8266.
Yang, H., Yan, R., Chen, H., Lee, D. H., & Zheng, C. (2007). Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel, 86(12/13), 1781–1788.
Sim, S. F., Mohamed, M., Lu, N. A., Lu, M. I., Sarman, N. S. P., & Samsudin, S. N. S. (2012). Computer-assisted analysis of fourier transform infrared (FTIR) spectra for characterization of various treated and untreated agriculture biomass. Bioresources, 7(4), 5367–5380.
Sprey, B. (1987). Complexity of cellulases from Trichoderma reesei with acidic isoelectric points: a two-dimensional gel electrophoretic study using immunoblotting. FEMS Microbiol Lett, 43, 25–32.
Leberknight, J., & Menkhaus, T. J. (2013). Membrane separations for solid–liquid clarification within lignocellulosic biorefining processes. Biotechnol Prog, 29(5), 1246–1254.
Gautam, A. K., & Menkhaus, T. J. (2014). Performance evaluation and fouling analysis for reverse osmosis and nanofiltration membranes during processing of lignocellulosic biomass hydrolysate. J Membr Sci, 451, 252–265.
Gurram, R. N., & Menkhaus, T. J. (2013). Analysis and characterization of heat transfer fouling during evaporation of a lignocellulosic biomass process stream. Ind Eng Chem Res, 52(32), 11111–11121.
Guarram, R. N., & Menkhaus, T. J. (2013). Effects of pH, slurry composition, and operating conditions on heat transfer fouling during evaporation of a lignocellulosic biomass process stream. Ind Eng Chem Res, 52(32), 11122–11131.
Acknowledgments
Financial support for R. Gurram was provided by the USDA NIFA, AFRI Competitive Grant No. 2010-65504-20372.
Conflict of Interest
No sources for conflicts of interest are present.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gurram, R.N., Menkhaus, T.J. Continuous Enzymatic Hydrolysis of Lignocellulosic Biomass with Simultaneous Detoxification and Enzyme Recovery. Appl Biochem Biotechnol 173, 1319–1335 (2014). https://doi.org/10.1007/s12010-014-0873-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0873-7