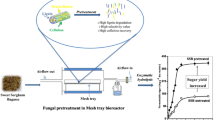
Abstract
Sweet sorghum (Sorghum sp.) has high biomass yield. Hydrolysis of lignocellulosic sweet sorghum bagasse (SSB) to fermentable sugar could be useful for manufacture of biofuel or other fermentation products. Pretreatment of lignocellulosic biomass to degrade lignin before enzymatic hydrolysis is a key step. Fungal pretreatment of SSB with combined CuSO4-gallic acid supplements in solid-state fermentation (SSF) to achieve higher lignin degradation, selectivity value (SV), and enzymatic hydrolysis to sugar was studied. Coriolus versicolor was selected due to high activities of ligninolytic enzymes laccase, lignin peroxidase (LiP), manganese peroxidase (MnP), polyphenol oxidase (PPO), and arylalcohol oxidase (AAO) and low activities of cellulolytic enzymes CMCase, FPase, and β-glucosidase with high lignin degradation and SV in 20 days. CuSO4/gallic acid increased the activities of ligninolytic enzymes resulting in enhanced lignin degradations and SVs. Cumulative/synergistic effect of combined supplements further increased the activities of laccase, LiP, MnP, PPO, and AAO by 7.6, 14.6, 2.67, 2.06, and 2.15-folds, respectively (than control), resulting in highest lignin degradation 31.1 ± 1.4% w/w (1.56-fold) and SV 2.33 (3.58-fold). Enzymatic hydrolysis of pretreated SSB yielded higher (~2.2 times) fermentable sugar. The study showed combined supplements can improve fungal pretreatment of lignocellulosic biomass. XRD, SEM, FTIR, and TGA/DTG of SSB confirmed the results.



Similar content being viewed by others
References
Ratnavathi, C. V., Chakravarthy, S. K., Komala, V. V., Chavan, U. D., & Patil, J. V. (2011). Sweet sorghum as feedstock for biofuel production: a review. Sugar Tech, 13, 399–407.
Hatakka, A., & Hammel, K. (2010). In: The Mycota; industrial applications, vol. 10 (Hofrichter, M., ed.), Springer, pp. 319–340.
Wan, C., & Li, Y. (2011). Effectiveness of microbial pretreatment by Ceriporiopsis subvermispora on different biomass feedstocks. Bioresource Technology, 102, 7507–7512.
Deswal, D., Gupta, R., Nandal, P., & Kuhad, R. C. (2014). Fungal pretreatment improves amenability of lignocellulosic material for its saccharification to sugars. Carbohydrate Polymers, 99, 264–269.
Zeng, J., Singh, D., & Chen, S. (2011). Biological pretreatment of wheat straw by Phanerochaete chrysosporium supplemented with inorganic salts. Bioresource Technology, 102, 3206–3214.
Tian, X. F., Fang, Z., & Guo, F. (2012). Impact and prospective of fungal pre-treatment of lignocellulosic biomass for enzymatic hydrolysis. Biofuels, Bioproducts and Biorefining, i, 1–16.
Hatakka, A. (1994). Lignin-modifying enzymes from selected white-rot fungi: production and role from in lignin degradation. FEMS Microbiology Reviews, 13, 125–135.
Liu, S., Wu, S. B., Pang, C. L., Li, W., & Dong, R. J. (2014). Microbial pretreatment of corn stovers by solid-state cultivation of Phanerochaete chrysosporium for biogas production. Appl Biochem Biotechnol, 1365–1376.
Zhi, Z., & Wang, H. (2014). White-rot fungal pretreatment of wheat straw with Phanerochaete chrysosporium for biohydrogen production: simultaneous saccharification and fermentation. Bioprocess and Biosystems Engineering, 37, 1447–1458.
Arora, A., Priya, S., Sharma, P., Sharma, S., & Nain, L. (2016). Evaluating biological pretreatment as a feasible methodology for ethanol production from paddy straw. Biocatalysis and Agricultural Biotechnology, 8, 66–72.
Asgher, M., Wahab, A., Bilal, M., & Iqbal, H. M. N. (2016). Lignocellulose degradation and production of lignin modifying enzymes by Schizophyllum commune IBL-06 in solid-state fermentation. Biocatalysis and Agricultural Biotechnology, 6, 195–201.
Rouches, E., Zhou, S., Steyer, J., & Carrere, H. (2016). White-rot fungi pretreatment of lignocellulosic biomass for anaerobic digestion: impact of glucose supplementation. Process Biochem.
Pamidipati, S., & Ahmed, A. (2016). Degradation of lignin in agricultural residues by locally isolated fungus Neurospora discreta. Applied Biochemistry and Biotechnology, 1–12.
Zeng, G., Cheng, M., Huang, D., Lai, C., Xu, P., Wei, Z., Li, N., Zhang, C., He, X., & He, Y. (2015). Study of the degradation of methylene blue by semi-solid-state fermentation of agricultural residues with Phanerochaete chrysosporium and reutilization of fermented residues. Waste Management, 38, 424–430.
Meehnian, H., & Jana, A. K. (2016). Cotton stalk pretreatment using Daedalea flavida, Phlebia radiata, and Flavodon flavus: lignin degradation, cellulose recovery, and enzymatic saccharification. Applied Biochemistry and Biotechnology, 1–20.
Bari, E., Nazarnezhad, N., Kazemi, S. M., Ghanbary, M. A. T., Mohebby, B., Schmidt, O., & Clausen, C. A. (2015). Comparison between degradation capabilities of the white rot fungi Pleurotus ostreatus and Trametes versicolor in beech wood. International Biodeterioration and Biodegradation, 104, 231–237.
Zhang, X., Yu, H., Huang, H., & Liu, Y. (2007). Evaluation of biological pretreatment with white rot fungi for the enzymatic hydrolysis of bamboo culms. Int. Biodeterior. Biodegradation, 60, 159–164.
Yu, H., Guo, G., Zhang, X., Yan, K., & Xu, C. (2009). The effect of biological pretreatment with the selective white-rot fungus Echinodontium taxodii on enzymatic hydrolysis of softwoods and hardwoods. Bioresource Technology, 100, 5170–5175.
Chang, A. J., Fan, J., & Wen, X. (2012). Screening of fungi capable of highly selective degradation of lignin in rice straw. International Biodeterioration and Biodegradation, 72, 26–30.
Salvachua, D., Prieto, A., Vaquero, M. E., Martínez, A. T., & Martínez, M. J. (2013). Sugar recoveries from wheat straw following treatments with the fungus Irpex lacteus. Bioresource Technology, 131, 218–225.
Meehnian, H., Jana, A. K., & Jana, M. M. (2017). Pretreatment of cotton stalks by synergistic interaction of Daedalea flavida and Phlebia radiata in co-culture for improvement in delignification and saccharification. International Biodeterioration and Biodegradation, 117, 68–77.
Shi, J., Sharma-Shivappa, R. R., Chinn, M., & Howell, N. (2009). Effect of microbial pretreatment on enzymatic hydrolysis and fermentation of cotton stalks for ethanol production. Biomass and Bioenergy, 33, 88–96.
Zhu, C., Bao, G., & Huang, S. (2016). Optimization of laccase production in the white-rot fungus Pleurotus ostreatus (ACCC 52857) induced through yeast extract and copper. Biotechnology & Biotechnological Equipment, 30, 270–276.
Song, L., Ma, F., Zeng, Y., Zhang, X., & Yu, H. (2013). The promoting effects of manganese on biological pretreatment with Irpex lacteus and enzymatic hydrolysis of corn stover. Bioresource Technology, 135, 89–92.
Sari, A. A., Yasin, H., Tachibana, S., & Hadibarata, T. (2016). Effects of mediators for ligninolytic enzyme production and kinetic studies on degradation of pentachlorobenzene by Trametes versicolor U80. Water, Air, & Soil Pollution, 227, 317.
Liu, Y., Sun, J., Luo, Z., Rao, S., Su, Y., & Yang, Y. (2013). Effect of supplements Mn2+, Cu2+, and aromatic compounds and Penicillium decumbens on lignocellulosic enzyme activity and productivity of Catathelasma ventricosum. Journal of Microbiology and Biotechnology, 23, 565–571.
Geiger, G., Brandl, H., Furrer, G., & Schulin, R. (1998). The effect of copper on the activity of cellulase and β-glucosidase in the presence of montmorillonite or Al-montmorillonite. Soil Biology and Biochemistry, 30, 1537–1544.
Tejirian, A., & Xu, F. (2010). Inhibition of cellulase-catalyzed lignocellulosic hydrolysis by iron and oxidative metal ions and complexes. Applied and Environmental Microbiology, 76, 7673–7682.
Pointing, S. B. (1999). Qualitative methods for the determination of lignocellulolytic enzyme production by tropical fungi. Fungal Diversity, 2, 17–33.
Teather, R. M., & Wood, P. J. (1982). Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Applied and Environmental Microbiology, 43, 777–780.
Archibald, F. S. (1992). A new assay for lignin-type peroxidases employing the dye azure B. Applied and Environmental Microbiology, 58, 3110–3116.
Bourbonnais, R., & Paice, M. G. (1990). Oxidation of non-phenolic substrates: an expanded role for laccase in lignin biodegradation. FEBS Letters, 267, 99–102.
Glenn, J. K., & Gold, M. H. (1985). Purification and characterization of an extracellular Mn (II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Archives of Biochemistry and Biophysics, 242, 329–341.
Wong, T. C., Luh, B. S., & Whitaker, J. R. (1971). Isolation and characterization of polyphenol oxidase isozymes of clingstone peach. Plant Physiology, 48, 19–23.
Guillen, F., Martinez, A. T., & Martinez, M. J. (1992). Substrate specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. European Journal of Biochemistry, 209, 603–611.
Ghose, T. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59, 257–268.
Wood, T. M., & Bhat, K. M. (1988). Methods for measuring cellulase activities. Methods in Enzymology, 160, 87–112.
Bailey, M. J., Biely, P., & Poutanen, K. (1992). Interlaboratory testing of methods for assay of xylanase activity. Journal of Biotechnology, 23, 257–270.
Aidoo, K. E., Hendry, R., & Wood, B. J. B. (1981). Estimation of fungal growth in a solid-state fermentation system. European Journal of Applied Microbiology and Biotechnology, 12, 6–9.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., & Crocker, D. (2010). Determination of structural carbohydrates and lignin in biomass: Laboratory Analytical Procedure (LAP). National Renewable Energy Laboratory, pp. NREL/TP 510 42618. Golden, Co: National Renewable Energy Laboratory, National Renewable Energy Laboratory.
Segal, L., Creely, J., Martin, A., & Conrad, C. (1959). An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Textile Research Journal, 29, 786–794.
Dowe, N., & McMillan, J. (2001). SSF experimental protocols: lignocellulosic biomass hydrolysis and fermentation: Laboratory Analytical Procedure (LAP). National Renewable Energy Laboratory (NREL) Analytical Procedures, pp. NREL/TP 510 42630. Golden, Co: National Renewable Energy Laboratory.
Taniguchi, M., Suzuki, H., Watanabe, D., Sakai, K., Hoshino, K., & Tanaka, T. (2005). Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. Journal of Bioscience and Bioengineering, 100, 637–643.
Zhang, X., Xu, C., & Wang, H. (2007). Pretreatment of bamboo residues with Coriolus versicolor for enzymatic hydrolysis. Journal of Bioscience and Bioengineering, 104, 149–151.
Knezevic, A., Milovanovic, I., Stajic, M., & Vukojevic, J. (2013). Potential of Trametes species to degrade lignin. Int. Biodeterior. Biodegradation, 85, 52–56.
Aguiar, A., Gavioli, D., & Ferraz, A. (2014). Metabolite secretion, Fe3+-reducing activity and wood degradation by the white-rot fungus Trametes versicolor ATCC 20869. Fungal Biology, 118, 935–942.
Gabhane, J., William, S. P., Vaidya, A. N., Das, S., & Wate, S. R. (2015). Solar assisted alkali pretreatment of garden biomass: effects on lignocellulose degradation, enzymatic hydrolysis, crystallinity and ultra-structural changes in lignocellulose. Waste Management, 40, 92–99.
Kaur, H., & Sudhakara, K. M. (2011). Effect of carbon, nitrogen sources and inducers on ligninolytic enzyme production by Morchella crassipes. World Journal of Microbiology and Biotechnology, 27, 687–691.
D’souza, T. M., Merritt, C. S., & Reddy, C. A. (1999). Lignin-modifying enzymes of the white rot basidiomycete Ganoderma lucidum. Applied and Environmental Microbiology, 65, 5307–5313.
Sun, S. L., Wen, J. L., Ma, M. G., Li, M. F., & Sun, R. C. (2013). Revealing the structural inhomogeneity of lignins from sweet sorghum stem by successive alkali extractions. Journal of Agricultural and Food Chemistry, 61, 4226–4235.
Acknowledgements
Mrs. Vartika Mishra gratefully acknowledges the Ministry of Human Resource Development (MHRD), Government of India for providing the fellowship during the study. All authors are highly thankful to the National Institute of Technology (NIT), Jalandhar for providing grants and administrative supports for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Supplementary Table 1
(DOCX 15 kb).
Supplementary Table 2
(DOCX 15 kb).
Supplementary Table 3
(DOCX 14 kb).
Supplementary Table 4
(DOCX 12 kb).
Supplementary Table 5
(DOCX 12 kb).
Supplementary Fig. 1
(DOCX 32 kb).
.
Supplementary Fig. 2
(DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Mishra, V., Jana, A.K. Fungal Pretreatment of Sweet Sorghum Bagasse with Combined CuSO4-Gallic Acid Supplement for Improvement in Lignin Degradation, Selectivity, and Enzymatic Saccharification. Appl Biochem Biotechnol 183, 200–217 (2017). https://doi.org/10.1007/s12010-017-2439-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2439-y