Abstract
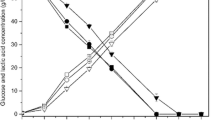
The availability of fermentable sugars in high concentrations in the sap of felled oil palm trunks and the thermophilic nature of the recently isolated Bacillus coagulans strain 191 were exploited for lactic acid production under non-sterile conditions. Screening indicated that strain 191 was active toward most sugars including sucrose, which is a major component of sap. Strain 191 catalyzed a moderate conversion of sap sugars to lactic acid (53%) with a productivity of 1.56 g/L/h. Pretreatment of oil palm sap (OPS) using alkaline precipitation improved the sugar fermentability, providing a lactic acid yield of 92% and productivity of 2.64 g/L/h. To better characterize potential inhibitors in the sap, phenolic, organic, and mineral compounds were analyzed using non-treated sap and saps treated with activated charcoal and alkaline precipitation. Phthalic acid, 3,4-dimethoxybenzoic acid, aconitic acid, syringic acid, and ferulic acid were reduced in the sap after treatment. High concentrations of Mg, P, K, and Ca were also precipitated by the alkaline treatment. These results suggest that elimination of excess phenolic and mineral compounds in OPS can improve the fermentation yield. OPS, a non-food resource that is readily available in bulk quantities from plantation sites, is a promising source for lactic acid production.

Similar content being viewed by others
References
Sumathi, S., Chai, S. P., & Mohamed, A. R. (2008). Utilization of oil palm as a source of renewable energy in Malaysia. Renewable and Sustainable Energy Reviews, 12, 2404–2421.
Agensi Inovasi Malaysia, (2012). National Biomass Strategy 2020: new wealth creation for Malaysia’s palm oil industry. Retrieved February 20, 2014 from http://www.feldaglobal.com/sitecontent/National%20Biomass%20Strategy%20Nov%202011%20FINAL.pdf.
Economics and Industry Development Division, Malaysian Palm Oil Board. Overview of The Malaysian Oil Palm Industry 2013. Retrieved February 26, 2014 http://bepi.mpob.gov.my/images/overview/Overview_of_Industry_2013.pdf.
Ng, F.-Y., Yew, F.-K., Basiron, Y., & Sundram, K. (2011). A renewable future driven with Malaysian palm oil-based green technology. J. Oil Palm Environ., 2, 1–7.
Kosugi, A., Tanaka, R., Magara, K., Murata, Y., Arai, T., Sulaiman, O., Hashim, R., Hamid, Z. A., Yahya, M. K., Yusof, M. N., Ibrahim, W. A., & Mori, Y. (2010). Ethanol and lactic acid production using sap squeezed from old oil palm trunks felled for replanting. Journal of Bioscience and Bioengineering, 110, 322–325.
Loh, Y. F., Md-Tahir, P., & Yeoh, B. H. (2011). Density distribution of oil palm stem veneer and its influence on plywood mechanical properties. Journal of Applied Sciences, 11, 824–831.
Mokhtar, A., Hassan, K., Aziz, A. A., & Wahid, M. B. (2011). Plywood from oil palm trunks. J. Oil Palm Res., 23, 1159–1165.
Chooklin, S., Kaewsichan, L., & Kaewsrichan, J. (2011). Potential use of Lactobacillus casei TISTR 1500 for the bioconversion from palmyra sap and oil palm sap to lactic acid. Electronic Journal of Biotechnology, 14, 1–13.
Payot, T., Chemaly, Z., & Fick, M. (1999). Lactic acid production by Bacillus coagulans-kinetic studies and optimization of culture medium for batch and continuous fermentations. Enzyme and Microbial Technology, 24, 191–199.
Ye, L., Hudari, M. S. B., Zhou, X., Zhang, D., Li, Z., & Wu, J. C. (2013). Conversion of acid hydrolysate of oil palm empty fruit bunch to L-lactic acid by newly isolated Bacillus coagulans JI12. Applied Microbiology and Biotechnology, 97, 4831–4838.
Sudesh, K., & Iwata, T. (2008). Sustainability of biobased and biodegradable plastics. CLEAN, 36, 433–442.
Martinez, F. A. C., Balciunas, E. M., Salgado, J. M., González, J. M. D., Converti, A., & Oliveira, R. P. S. (2013). Lactic acid properties, applications and production: a review. Trends. Food. Sci. Tech., 30, 70–83.
Lunt, J. (1998). Large-scale production, properties and commercial applications of polylactic acid polymers. Polymer Degradation and Stability, 59, 145–152.
Qin, J., Zhao, B., Wang, X., Wang, L., Yu, B., Ma, Y., Ma, C., Tang, H., Sun, J., & Xu, P. (2009). Non-sterilized fermentative production of polymer-grade l-lactic acid by a newly isolated thermophilic strain Bacillus sp. 2–6. PloS One, 4, e4359.
Dumbrepatil, A., Adsul, M., Chaudhari, S., Khire, J., & Gokhale, D. (2008). Utilization of molasses sugar for lactic acid production by Lactobacillus delbrueckii subsp. delbrueckii mutant Uc-3 in batch fermentation. Applied and Environmental Microbiology, 74, 333–335.
Zhang, Z. Y., Jin, B., & Kelly, J. M. (2007). Production of lactic acid and byproducts from waste potato starch by Rhizopus arrhizus: role of nitrogen sources. World Journal of Microbiology and Biotechnology, 23, 229–236.
Jawad, A. H., Alkarkhi, A. F. M., Jason, O. C., Easa, A. M., & Nik Norulaini, N. A. (2013). Production of the lactic acid from mango peel waste—factorial experiment. J. King Saud Univ. Sci., 25, 39–45.
Michelson, T., Kask, K., Jõgia, E., Talpsep, E., Suitso, I., & Nurk, A. (2006). L(+)-lactic acid producer Bacillus coagulans SIM-7 DSM 14043 and its comparison with Lactobacillus delbrueckii ssp. lactis DSM 20073. Enzyme and Microbial Technology, 39, 861–867.
Rhee, M. S., Moritz, B. E., Xie, G., Glavina Del Rio, T., Dalin, E., Tice, H., Bruce, D., Goodwin, L., Chertkov, O., Brettin, T., Han, C., Detter, C., Pitluck, S., Land, M. L., Patel, M., Ou, M., Harbrucker, R., Ingram, L. O., & Shanmugam, K. T. (2011). Complete genome sequence of a thermotolerant sporogenic lactic acid bacterium, Bacillus coagulans strain 36D1. Standards in Genomic Sciences, 5, 331–340.
Ye, L., Zhou, X., Hudari, M. S. B., Li, Z., & Wu, J. C. (2013). Highly efficient production of L-lactic acid from xylose by newly isolated Bacillus coagulans C106. Bioresource Technology, 132, 38–44.
Singleton, V. L., & Rossi Jr., J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol.Vitic., 16, 144–158.
Hofvendahl, K., & Hahn-Hägerdal, B. (2000). Factors affecting the fermentative lactic acid production from renewable resources. Enzyme and Microbial Technology, 26, 87–107.
Bastidas-Oyanedel, J. R., Fang, C., Almardeai, S., Javid, U., Yousuf, A., & Schmidt, J. E. (2016). Waste biorefinery in arid/semi-arid regions. Bioresource Technology, 215, 21–28.
Hujanen, M., & Linko, Y. Y. (1996). Effect of temperature and various nitrogen sources on L(+)-lactic acid production by Lactobacillus casei. Applied Microbiology and Biotechnology, 45, 307–313.
Wang, L., Zhao, B., Liu, B., Yu, B., Ma, C., Su, F., Hua, D., Li, Q., Ma, Y., & Xu, P. (2010). Efficient production of L-lactic acid from corncob molasses, a waste by-product in xylitol production, by a newly isolated xylose utilizing Bacillus sp. strain. Bioresource Technology, 101, 7908–7915.
Eiteman, M. A., Lee, S. A., Altman, R., & Altman, E. (2009). A substrate-selective co-fermentation strategy with Escherichia coli produces lactate by simultaneously consuming xylose and glucose. Biotechnology and Bioengineering, 102, 822–827.
Alriksson, B. (2006). Ethanol from lignocellulose: alkali detoxification of dilute-acid spruce hydrolysates (p. 28). Karlstad: Faculty of Technology and Science Biochemistry. Karlstads Universitet.
Mussatto, S. I., & Roberto, I. C. (2004). Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresource Technology, 93, 1–10.
Palmqvist, E., & Hahn-Hägerdal, B. (2000). Fermentation of lignocellulosic hydrolysates. 1: inhibition and detoxification. Bioresource Technology, 74, 17–24.
Alriksson, B., Sjöde, A., Nilvebrant, N.-O., & Jönsson, L. J. (2006). Optimal conditions for alkaline detoxification of dilute-acid lignocellulose hydrolysates. Applied Biochemistry and Biotechnology, 130, 599–611.
Kamal, S. M. M., Mohamad, N. L., Abdullah, A. G. L., & Abdullah, N. (2011). Detoxification of sago trunk hydrolysate using activated charcoal for xylitol production. Proced. Food Sci., 1, 908–913.
Soto, M. L., Moure, A., Domínguez, H., & Parajó, J. C. (2011). Recovery, concentration and purification of phenolic compounds by adsorption: a review. Journal of Food Engineering, 105, 1–27.
Larsson, S., Reimann, A., Nilvebrant, N.-O., & Jönsson, L. J. (1999). Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Applied Biochemistry and Biotechnology, 77, 91–103.
Karmakar, B., Vohra, R. M., Nandanwar, H., Sharma, P., Gupta, K. G., & Sobti, R. C. (2000). Rapid degradation of ferulic acid via 4-vinylguaiacol and vanillin by a newly isolated strain of Bacillus coagulans. Journal of Biotechnology, 80, 195–202.
Sadler, W. R., & Trudinger, P. A. (1967). The inhibition of microorganisms by heavy metals. Mineralium Deposita, 2, 158–168.
John, R., Nampoothiri, K. M., & Pandey, A. (2007). Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Applied Microbiology and Biotechnology, 74, 524–534.
Acknowledgements
B. Kunasundari acknowledges the Japan International Research Center for Agricultural Sciences (JIRCAS) Fellowship for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Pretreatment of oil palm sap for efficient lactic acid production.
• Alkaline pretreatment was effective in removing fermentation inhibitors from sap.
• A lactic acid yield of 92% was obtained from alkaline-pretreated sap.
Rights and permissions
About this article
Cite this article
Kunasundari, B., Arai, T., Sudesh, K. et al. Detoxification of Sap from Felled Oil Palm Trunks for the Efficient Production of Lactic Acid. Appl Biochem Biotechnol 183, 412–425 (2017). https://doi.org/10.1007/s12010-017-2454-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2454-z