Abstract
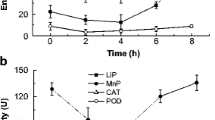
In this study, selenium (Se) induction of the ligninolytic enzyme manganese-dependent peroxidase (MnP) production, and the effects on the oxidative state in the white-rot fungus Bjerkandera adusta (Willdenow) P. Karsten were demonstrated. Low concentration of Se (0.5 mM) caused up to a twofold increase in MnP production (0.81 ± 0.05 U/ml) when compared to control (0.39 ± 0.07 U/ml), whereas higher concentrations of Se (200 mM) inhibited (0.03 ± 0.01 U/ml) MnP production. Addition of high concentration of Se also caused up to a twofold increase in lipid peroxidation levels. These results demonstrate for the first time that Se may induce or reduce MnP production and lipid peroxidation levels which play a significant role in lignin degradation by white-rot fungi.



Similar content being viewed by others
Abbreviations
- Ag:
-
Silver
- Cd:
-
Cadmium
- Cu:
-
Copper
- Hg:
-
Mercury
- Lac:
-
laccase
- LiP:
-
lignin peroxidase
- MDA:
-
Malondialdehyde
- Mn:
-
Manganese
- MnP:
-
Manganese-dependent peroxidase
- Se:
-
Selenium
- Zn:
-
Zinc
References
Brigham JS, Adney WS, Himmel ME (1996) Hemicelluloses: diversity and applications. In: Wyman CE (ed) Handbook on bioethanol: production and utilization. Taylor and Francis, Washington, DC, USA, pp 119–142
Wesenberg D, Kyriakides I, Agathos SN (2003) White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotech Adv 22:161–187
Novotny C, Svobodova K, Erbanova P, Cajthaml T, Kasinath A, Lang E, Šašek V (2004) Ligninolytic fungi in bioremediation: extracellular enzyme production and degradation rate. Soil Biol Biochem 36:1545–1551
Fenice M, Giovannozzi SG, Federici F, D'Annibale A (2003) Submerged and solid-state production of laccase and Mn-peroxidase by Panus tigrinus on olive mill wastewater-based media. J Biotechnol 100:77–85
Dominguez A, Rivela I, Couto SR, Sanromán MA (2001) Design of a new rotating drum bioreactor for ligninolytic enzyme production by Phanerochaete chrysosporium grown on an inert support. Process Biochem 37:549–554
Nakamura Y, Godliving-Sungusia M, Sawada T, Kuwahara M (1999) Lignin-degrading enzyme production by Bjerkandera adusta immobilized on polyurethane foam. J Biosci Bioeng 88:41–47
Hatakka A (1994) Lignin modifying enzymes from selected white-rot fungi: production and role in lignin degradation. FEMS Microbiol Rev 13:125–135
Bermek H, Gülseren İ, Li K, Jung H, Tamerler C (2004) The effect of fungal morphology on ligninolytic enzyme production by a recently isolated wood-degrading fungus Trichophyton rubrum LSK-27. World J Mic Biotech 20:345–349
Howlett NG, Avery SV (1997) Induction of lipid peroxidation during heavy metal stress in Saccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturation. Appl Environ Microbiol 63:2971–2976
Baldrian P (2003) Interactions of heavy metals with white-rot fungi. Enzyme Microb Technol 32:78–91
Baldrian P, Gabriel J (2002) Copper and cadmium increase laccase activity in Pleurotus ostreatus. FEMS Microbiol Lett 206:69–74
Collins PJ, Dobson ADW (1997) Regulation of laccase gene transcription in Trametes versicolor. Appl Environ Microbiol 63:3444–3450
Munoz AHS, Kubachka K, Wrobel K, Corona JFG, Yathavakilla SKV, Caruso JA, Wrobel K (2006) Se-enriched mycelia of Pleurotus ostreatus: distribution os selenium in cell walls and cell membranes/cytosol. J Agric Food Chem 54:3440–3444
Tapiero H, Townsend DM, Tew KD (2003) The antioxidant role of selenium and seleno-compounds. Biomed Pharmacother 57:134–144
Ledwozyw A, Michalak J, Stepien A, Kadziolka A (1986) The relationship between plasma tryglicerides, cholesterol, total lipids and lipid peroxidation products during human atherosclerosis. Clinica Chimica Acta 155:275–284
Galhaup C, Haltrich D (2001) Enhanced formation of laccase activity by the white-rot fungus Trametes pubescens in the presence of copper. Appl Microbiol Biotechnol 56:225–232
Baldrian P, Valaskova V, Merhautova V, Gabriel J (2005) Degradation of lignocellulose by Pleurotus ostreatus in the presence of copper, manganese, lead and zinc. Res Microbiol 156:670–676
Levin L, Forchiassin F, Papinutti L (2002) Effect of copper on the ligninolytic activity of Trametes trogii. Int Biodeterior Biodegrad 49:60
Enoki M, Watanabe T, Nakagame S, Koller K, Messner K, Honda Y, Kuwahara M (1999) Extracellular lipid peroxidation of selective white-rot fungus, Ceriporiopsis subvermispora. FEMS Microbiol Lett 180:205–211
Ginkel VG, Sevian A (1994) Lipid peroxidation-induced membrane structural alterations. Methods Enzymol 233:273–288
Hammel KE, Kapich AN, Jensen JKA, Ryan ZC (2002) Reactive oxygen species as agents of wood decay by fungi. Enz Microbiol Tech 30:445–453
Kapich AN, Prior BA, Lundell T, Hatakka A (2005) A rapid method to quantify pro-oxidant activity in cultures of wood-decaying white-rot fungi. J Microbiol Methods 61:261–271
Acknowledgments
This work was funded by Turkish State Planning Organization project titled “Advanced Technologies in Engineering”. We would also like to thank Pinar Huner for her valuable help in MnP stability studies. The authors also thank Kelsey Fisher for her help in proof correction.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Catal, T., Liu, H. & Bermek, H. Selenium Induces Manganese-dependent Peroxidase Production by the White-Rot Fungus Bjerkandera adusta (Willdenow) P. Karsten. Biol Trace Elem Res 123, 211–217 (2008). https://doi.org/10.1007/s12011-007-8084-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-007-8084-5