Abstract
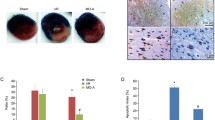
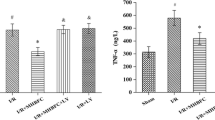
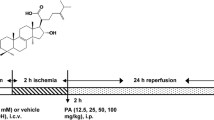
Cordycepin (3′-deoxyadenosine) isolated from Cordyceps militaris, a species of the fungal genus Cordyceps, has been shown to exhibit many pharmacological functions, such as anticancer, anti-inflammatory, and antioxidant activities. In this study, we investigated the preventive role of cordycepin in ischemic/reperfusion (I/R) injury of isolated rat hearts and anesthetized rats. After Sprague–Dawley rats received cordycepin (3, 10, and 30 mg/kg) or control (0.5 % carboxyl methylcellulose) orally once a day for a week, hearts were isolated and mounted on Langendorff heart perfusion system. Isolated hearts were perfused with Krebs–Henseleit buffer for 15-min pre-ischemic stabilization period and subjected to 30-min global ischemia and 30-min reperfusion. Cordycepin administration (10 mg/kg, p.o.) significantly increased left ventricular developed pressure during the reperfusion period compared to that in the control group, but without any effect on coronary flow. Cordycepin (10 mg/kg, p.o.) significantly increased the phosphorylation of Akt/GSK-3β/p70S6K pathways, which are known to modulate multiple survival pathways. In addition, cordycepin decreased Bax and cleaved caspase-3 expression while increasing Bcl-2 expression, Bcl-2/Bax ratio, and heme oxygenase (HO-1) expression in isolated rat hearts. In anesthetized rats subjected to 30 min occlusion of left anterior descending coronary artery/2.5-h reperfusion, cordycepin (1, 3, and 10 mg/kg, i.v.) administered 15 min before the onset of ischemia dose-dependently decreased the infarct size in left ventricle. In conclusion, cordycepin could be an attractive therapeutic candidate with oral activity against I/R-associated heart diseases such as myocardial infarction.





Similar content being viewed by others
Abbreviations
- CF:
-
Coronary flow
- CMC:
-
Carboxyl methylcellulose
- ERK:
-
Extracellular signal-regulated kinase
- LVDL:
-
Left ventricular developed pressure
- LVEDP:
-
Left ventricular end-diastolic pressure
- XTT:
-
2,3-Bis [2-methyloxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide
- PVDF:
-
Polyvinylidene fluoride
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Murphy, E., & Steenbergen, C. (2008). Mechanisms underlying acute protection from cardiac ischemia–reperfusion injury. Physiological Reviews, 88, 581–609.
Buja, L. M. (2005). Myocardial ischemia and reperfusion injury. Cardiovascular Pathology, 14, 170–175.
Ambrosio, G., Zweier, J. L., Duilio, C., Kuppusamy, P., Santoro, G., Elia, P. P., et al. (1993). Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. Journal of Biological Chemistry, 268, 18532–18541.
Griendling, K. K., & FitzGerald, G. A. (2003). Oxidative stress and cardiovascular injury: Part I: Basic mechanisms and in vivo monitoring of ROS. Circulation, 108, 1912–1916.
Marczin, N., El-Habashi, N., Hoare, G. S., Bundy, R. E., & Yacoub, M. (2003). Antioxidants in myocardial ischemia–reperfusion injury: Therapeutic potential and basic mechanisms. Archives of Biochemistry and Biophysics, 420, 222–236.
Ryter, S. W., Morse, D., & Choi, A. M. (2007). Carbon monoxide and bilirubin: Potential therapies for pulmonary/vascular injury and disease. American Journal of Respiratory Cell and Molecular Biology, 36, 175–182.
Abraham, N. G., & Kappas, A. (2008). Pharmacological and clinical aspects of heme oxygenase. Pharmacological Reviews, 60, 79–127.
Cunningham, K. G., Manson, W., Spring, F. S., & Hutchinson, S. A. (1950). Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature, 166, 949.
Feng, X. (2005). Regulatory roles and molecular signaling of TNF family members in osteoclasts. Gene, 350, 1–13.
Won, K. J., Lee, S. C., Lee, C. K., Lee, H. M., Lee, S. H., Fang, Z., et al. (2009). Cordycepin attenuates neointimal formation by inhibiting reactive oxygen species-mediated responses in vascular smooth muscle cells in rats. Journal of Pharmacological Sciences, 109, 403–412.
Cheng, Z., He, W., Zhou, X., Lv, Q., Xu, X., Yang, S., et al. (2011). Cordycepin protects against cerebral ischemia/reperfusion injury in vivo and in vitro. European Journal of Pharmacology, 664, 20–28.
Yang, M. K., Lee, S. H., Seo, H. W., Yi, K. Y., Yoo, S. E., Lee, B. H., et al. (2009). KR-31761, a novel K+(ATP)-channel opener, exerts cardioprotective effects by opening both mitochondrial K+(ATP) and Sarcolemmal K+(ATP) channels in rat models of ischemia/reperfusion-induced heart injury. Journal of Pharmacological Sciences, 109, 222–232.
Das, A., Xi, L., & Kukreja, R. C. (2008). Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. Journal of Biological Chemistry, 283, 29572–29585.
Kaga, S., Zhan, L., Altaf, E., & Maulik, N. (2006). Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium. Journal of Molecular and Cellular Cardiology, 40, 138–147.
Cheng, Z., Surichan, S., Ruparelia, K., Arroo, R., & Boarder, M. R. (2011). Tangeretin and its metabolite 4′-hydroxytetramethoxyflavone attenuate EGF-stimulated cell cycle progression in hepatocytes; role of inhibition at the level of mTOR/p70S6K. British Journal of Pharmacology, 162, 1781–1791.
Sugden, P. H. (2003). Ras, Akt, and mechanotransduction in the cardiac myocyte. Circulation Research, 93, 1179–1192.
Menon, B., Johnson, J. N., Ross, R. S., Singh, M., & Singh, K. (2007). Glycogen synthase kinase-3beta plays a pro-apoptotic role in beta-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes: Role of beta1 integrins. Journal of Molecular and Cellular Cardiology, 42, 653–661.
Sugden, P. H., Fuller, S. J., Weiss, S. C., & Clerk, A. (2008). Glycogen synthase kinase 3 (GSK3) in the heart: A point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. British Journal of Pharmacology, 153(Suppl 1), S137–S153.
Das, D. K., & Maulik, N. (2004). Conversion of death signal into survival signal by redox signaling. Biochemistry (Mosc), 69, 10–17.
Zhao, Z. Q., Corvera, J. S., Halkos, M. E., Kerendi, F., Wang, N. P., Guyton, R. A., et al. (2003). Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. American Journal of Physiology Heart and Circulatory Physiology, 285, H579–H588.
Tsang, A., Hausenloy, D. J., Mocanu, M. M., & Yellon, D. M. (2004). Postconditioning: A form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circulation Research, 95, 230–232.
Yang, X. M., Philipp, S., Downey, J. M., & Cohen, M. V. (2005). Postconditioning’s protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Research in Cardiology, 100, 57–63.
Miyamoto, S., Murphy, A. N., & Brown, J. H. (2009). Akt mediated mitochondrial protection in the heart: Metabolic and survival pathways to the rescue. Journal of Bioenergetics and Biomembranes, 41, 169–180.
Das, D. K., & Maulik, N. (2005). Mitochondrial function in cardiomyocytes: Target for cardioprotection. Current Opinion in Anesthesiology, 18, 77–82.
Horbinski, C., & Chu, C. T. (2005). Kinase signaling cascades in the mitochondrion: A matter of life or death. Free Radical Biology and Medicine, 38, 2–11.
Juhaszova, M., Zorov, D. B., Kim, S. H., Pepe, S., Fu, Q., Fishbein, K. W., et al. (2004). Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. Journal of Clinical Investigation, 113, 1535–1549.
Javadov, S., & Karmazyn, M. (2007). Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cellular Physiology and Biochemistry, 20, 1–22.
Haq, S., Choukroun, G., Kang, Z. B., Ranu, H., Matsui, T., Rosenzweig, A., et al. (2000). Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy. Journal of Cell Biology, 151, 117–130.
Barillas, R., Friehs, I., Cao-Danh, H., Martinez, J. F., & del Nido, P. J. (2007). Inhibition of glycogen synthase kinase-3beta improves tolerance to ischemia in hypertrophied hearts. Annals of Thoracic Surgery, 84, 126–133.
Rajak, S., Banerjee, S. K., Sood, S., Dinda, A. K., Gupta, Y. K., Gupta, S. K., et al. (2004). Emblica officinalis causes myocardial adaptation and protects against oxidative stress in ischemic–reperfusion injury in rats. Phytotherapy Research, 18, 54–60.
Cao, J., Drummond, G., Inoue, K., Sodhi, K., Li, X. Y., & Omura, S. (2008). Upregulation of heme oxygenase-1 combined with increased adiponectin lowers blood pressure in diabetic spontaneously hypertensive rats through a reduction in endothelial cell dysfunction, apoptosis and oxidative stress. International Journal of Molecular Sciences, 9, 2388–2406.
Zweier, J. L., & Talukder, M. A. (2006). The role of oxidants and free radicals in reperfusion injury. Cardiovascular Research, 70, 181–190.
Elmarakby, A. A., Faulkner, J., Posey, S. P., & Sullivan, J. C. (2010). Induction of hemeoxygenase-1 attenuates the hypertension and renal inflammation in spontaneously hypertensive rats. Pharmacological Research, 62, 400–407.
Hsu, J. T., Kan, W. H., Hsieh, C. H., Choudhry, M. A., Bland, K. I., & Chaudry, I. H. (2009). Mechanism of salutary effects of estrogen on cardiac function following trauma-hemorrhage: Akt-dependent HO-1 up-regulation. Critical Care Medicine, 37, 2338–2344.
Pachori, A. S., Smith, A., McDonald, P., Zhang, L., Dzau, V. J., & Melo, L. G. (2007). Heme-oxygenase-1-induced protection against hypoxia/reoxygenation is dependent on biliverdin reductase and its interaction with PI3K/Akt pathway. Journal of Molecular and Cellular Cardiology, 43, 580–592.
Zhou, X., Meyer, C. U., Schmidtke, P., & Zepp, F. (2002). Effect of cordycepin on interleukin-10 production of human peripheral blood mononuclear cells. European Journal of Pharmacology, 453, 309–317.
Chen, L. S., Stellrecht, C. M., & Gandhi, V. (2008). RNA-directed agent, cordycepin, induces cell death in multiple myeloma cells. British Journal of Haematology, 140, 391–682.
Acknowledgments
This study was supported by the Konkuk University.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eun-Seok Park and Do-Hyun Kang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Park, ES., Kang, DH., Yang, MK. et al. Cordycepin, 3′-Deoxyadenosine, Prevents Rat Hearts from Ischemia/Reperfusion Injury Via Activation of Akt/GSK-3β/p70S6K Signaling Pathway and HO-1 Expression. Cardiovasc Toxicol 14, 1–9 (2014). https://doi.org/10.1007/s12012-013-9232-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-013-9232-0