Abstract
Here we examine the structure of the various types of spine synapses throughout the animal kingdom. Based on available evidence, we suggest that there are two major categories of spine synapses: invaginating and non-invaginating, with distributions that vary among different groups of animals. In the simplest living animals with definitive nerve cells and synapses, the cnidarians and ctenophores, most chemical synapses do not form spine synapses. But some cnidarians have invaginating spine synapses, especially in photoreceptor terminals of motile cnidarians with highly complex visual organs, and also in some mainly sessile cnidarians with rapid prey capture reflexes. This association of invaginating spine synapses with complex sensory inputs is retained in the evolution of higher animals in photoreceptor terminals and some mechanoreceptor synapses. In contrast to invaginating spine synapse, non-invaginating spine synapses have been described only in animals with bilateral symmetry, heads and brains, associated with greater complexity in neural connections. This is apparent already in the simplest bilaterians, the flatworms, which can have well-developed non-invaginating spine synapses in some cases. Non-invaginating spine synapses diversify in higher animal groups. We also discuss the functional advantages of having synapses on spines and more specifically, on invaginating spines. And finally we discuss pathologies associated with spine synapses, concentrating on those systems and diseases where invaginating spine synapses are involved.


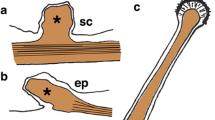
The micrograph in a2 is figure 3B from Gray et al. (2009; Biol. Bull. 217:35–49), reprinted with permission from the Marine Biological Laboratory, Woods Hole, MA (and from Dr. R.A. Satterlie); that in d2 is a reprint of Figure 11 from Holmberg (1970), with permission from Springer Publishing Company (Color figure online)

The micrograph in a2 is a reprint of Figure 2D from Budelmann and Thies (1977) with permission from Springer Publishing Company (Color figure online)






Similar content being viewed by others
References
Achatz, J. G., & Martinez, P. (2012). The nervous system of Isodiametra pulchra (Acoela) with a discussion on the neuroanatomy of the Xenacoelomorpha and its evolutionary implications. Frontiers in Zoology, 9, 27.
Acker, C. D., Yan, P., & Loew, L. M. (2011). Single-voxel Recording of voltage transients in dendritic spines. Biophysical Journal, 101(2), L11–L13.
Acsády, L., Kamondi, A., Sik, A., Freund, T., & Buzsaki, G. (1998). GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. Journal of Neuroscience, 18(9), 3386–3403.
Akert, K., Pfenning, K., & Sandri, C. (1967a). Fine structure of synapses in subfornical organ of cat. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie, 81(4), 537–556.
Akert, K., Pfenninger, K., & Sandri, C. (1967b). Crest synapses with subjunctional bodies in the subfornical organ. Brain Research, 5(1), 118–120.
Alberstein, R., Grey, R., Zimmet, A., Simmons, D. K., & Mayer, M. L. (2015). Glycine activated ion channel subunits encoded by ctenophore glutamate receptor genes. Proceedings of the National Academy of Sciences of the United States of America, 112(44), E6048–E6057.
Albertson, D. G., & Thomson, J. N. (1976). Pharynx of caenorhabditis elegans. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 275(938), 299–325.
Altman, J., & Bayer, S. A. (1997). Development of the cerebellar system in relation to its evolution, structure, and functions. New York: CRC Press.
Alvarez-Otero, R., Regueira, S. D., & Anadon, R. (1993). New structural aspects of the synaptic contacts on Purkinje cells in an elasmobranch cerebellum. Journal of Anatomy, 182(Pt 1), 13–21.
Anderson, P. A. V., & Grunert, U. (1988). 3-Dimensional structure of bidirectional, excitatory chemical Synapses in the jellyfish Cyanea-Capillata. Synapse, 2(6), 606–613.
Araya, R., Jiang, J., Eisenthal, K. B., & Yuste, R. (2006). The spine neck filters membrane potentials. Proceedings of the National Academy of Sciences of the United States of America, 103(47), 17961–17966.
Arendt, D., Tosches, M. A., & Marlow, H. (2016). From nerve net to nerve ring, nerve cord and brain - evolution of the nervous system. Nature Reviews Neuroscience, 17, 61–72.
Arluison, M., & de la Manche, I. S. (1980). High-resolution radioautographic study of the serotonin innervation of the rat corpus striatum after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience, 5(2), 229–240.
Aronin, N., DiFiglia, M., Liotta, A. S., & Martin, J. B. (1981). Ultrastructural localization and biochemical features of immunoreactive LEU-enkephalin in monkey dorsal horn. Journal of Neuroscience, 1(6), 561–577.
Ashby, M. C., Maier, S. R., Nishimune, A., & Henley, J. M. (2006). Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. Journal of Neuroscience, 26(26), 7046–7055.
Babb, T. L., Kupfer, W. R., Pretorius, J. K., Crandall, P. H., & Levesque, M. F. (1991). Synaptic reorganization by mossy fibers in Human epileptic Fascia-Dentata. Neuroscience, 42(2), 351–363.
Bailey, C. H., Kandel, E. R., & Harris, K. M. (2015). Structural components of synaptic plasticity and memory consolidation. Cold Spring Harbor Perspectives in Biology, 7, a021758. doi:10.1101/cshperspect.a021758.
Bailey, C. H., & Thompson, E. B. (1979). Indented synapses in Aplysia. Brain Research, 173(1), 13–20.
Bailey, C. H., Thompson, E. B., Castellucci, V. F., & Kandel, E. R. (1979). Ultrastructure of the synapses of sensory neurons that mediate the Gill-withdrawal reflex in Aplysia. Journal of Neurocytology, 8(4), 415–444.
Baker, C. A., Montey, K. L., Pongstaporn, T., & Ryugo, D. K. (2010). Postnatal development of the endbulb of held in congenitally deaf cats. Front Neuroanatical, 4, 19.
Bedini, C., & Lanfranchi, A. (1991). The central and peripheral nervous-system of Acoela (Plathelminthes). An electron-microscopic study. Acta Zoologica, 72(2), 101–106.
Bedini, C., & Lanfranchi, A. (1998). Ultrastructural study of the brain of a typhloplanid flatworm. Acta Zoologica, 79(3), 243–249.
Bell, C. C., Han, V., & Sawtell, N. B. (2008). Cerebellum-like structures and their implications for cerebellar function. Annual Review of Neuroscience, 31, 1–24.
Bellot, A., Guivernau, B., Tajes, M., Bosch-Morato, M., Valls-Comamala, V., & Munoz, F. J. (2014). The structure and function of actin cytoskeleton in mature glutamatergic dendritic spines. Brain Research, 1573, 1–16.
Benarroch, E. E. (2008). Suprachiasmatic nucleus and melatonin reciprocal interactions and clinical correlations. Neurology, 71(8), 594–598.
Benwitz, G. (1978). Electron-microscopic investigations on development of colloblasts in Ctenophore Pleurobrachia-Pileus (Tentaculifera, Cydippea). Zoomorphologie, 89(3), 257–278.
Bergson, C., Mrzljak, L., Smiley, J. F., Pappy, M., Levenson, R., & Goldman-Rakic, P. S. (1995). Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. Journal of Neuroscience, 15(12), 7821–7836.
Berson, E. L. (1993). Retinitis-Pigmentosa. The Friedenwald Lecture. Investigative Ophthalmology & Visual Science, 34(5), 1659–1676.
Bery, A., Cardona, A., Martinez, P., & Hartenstein, V. (2010). Structure of the central nervous system of a juvenile acoel. Symsagittifera roscoffensis. Development Genes and Evolution, 220(3–4), 61–76.
Bischofberger, J., Engel, D., Frotscher, M., & Jonas, P. (2006). Timing and efficacy of transmitter release at mossy fiber synapses in the hippocampal network. Pflugers Archiv-European Journal of Physiology, 453(3), 361–372.
Blanks, J. C., Adinolfi, A. M., & Lolley, R. N. (1974). Synaptogenesis in the photoreceptor terminal of the mouse retina. Journal of Comparative Neurology, 156(1), 81–93.
Bloodgood, B. L., Giessel, A. J., & Sabatini, B. L. (2009). Biphasic synaptic Ca influx arising from compartmentalized electrical signals in dendritic spines. PLoS Biology, 7(9), e1000190. doi:10.1371/journal.pbio.1000190.
Borst, A., & Helmstaedter, M. (2015). Common circuit design in fly and mammalian motion vision. Nature Neuroscience, 18(8), 1067–1076.
Bosch, M., Castro, J., Saneyoshi, T., Matsuno, H., Sur, M., & Hayashi, Y. (2014). Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron, 82(2), 444–459.
Boschek, C. B. (1971). Fine structure of peripheral retina and Lamina Ganglionaris of fly, Musca-Domestica. zeitschrift Fur Zellforschung Und Mikroskopische Anatomie, 118(3), 369–409.
Bourne, J. N., & Harris, K. M. (2008). Balancing structure and function at hippocampal dendritic spines. Annual Review of Neuroscience, 31, 47–67.
Boycott, B. B., Guillery, R. W., & Gray, E. G. (1961). Synaptic structure and its alteration with environmental temperature: A study by light and electron microscopy of central nervous system of lizards. Proceedings of the Royal Society Series B-Biological Sciences, 154(955), 151+.
Brandon, J. G., & Coss, R. G. (1982). Rapid dendritic spine stem shortening during one-trial learning: the honeybee’s first orientation flight. Brain Research, 252(1), 51–61.
Brown, S., & Wolff, G. (2012). Fine structural organization of the hemiellipsoid body of the land hermit crab, Coenobita clypeatus. Journal of Comparative Neurology, 520(13), 2847–2863.
Budelmann, B. U., Sachse, M., & Staudigl, M. (1987). The angular-acceleration receptor system of the statocyst of Octopus-Vulgaris: Morphometry, ultrastructure, and neuronal and synaptic organization. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 315(1174), 305–343.
Budelmann, B. U., & Thies, G. (1977). Secondary sensory cells in gravity receptor system of statocyst of Octopus-Vulgaris. Cell and Tissue Research, 182(1), 93–98.
Burighel, P., Lane, N. J., Fabio, G., Stefano, T., Zaniolo, G., Carnevali, M. D., & Manni, L. (2003). Novel, secondary sensory cell organ in ascidians: in search of the ancestor of the vertebrate lateral line. Journal of Comparative Neurology, 461(2), 236–249.
Buttarelli, F. R., Pellicano, C., & Pontieri, F. E. (2008). Neuropharmacology and behavior in planarians: Translations to mammals. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology, 147(4), 399–408.
Byzov, A. L., & Shura-Bura, T. M. (1986). Electrical feedback mechanism in the processing of signals in the outer plexiform layer of the retina. Vision Research, 26(1), 33–44.
Campellone, K. G., & Leong, J. M. (2003). Tails of two Tirs: actin pedestal formation by enteropathogenic E. coli and enterohemorrhagic E. coli O157:H7. Current Opinion in Microbiology, 6(1), 82–90.
Cannon, J. T., Vellutini, B. C., Smith, J, 3rd, Ronquist, F., Jondelius, U., & Hejnol, A. (2016). Xenacoelomorpha is the sister group to Nephrozoa. Nature, 530(7588), 89–93.
Cano, J., Pasik, P., & Pasik, T. (1989). Early postnatal development of the monkey globus pallidus: A golgi and electron microscopic study. Journal of Comparative Neurology, 279(3), 353–367.
Carr, D. B., & Sesack, S. R. (1996). Hippocampal afferents to the rat prefrontal cortex: Synaptic targets and relation to dopamine terminals. Journal of Comparative Neurology, 369(1), 1–15.
Case, N. M., Young, J. Z., & Gray, E. G. (1972). Ultrastructure and synaptic relations in optic lobe of brain of Eledone and Octopus. Journal of Ultrastructure Research, 39(1–2), 115–123.
Castejón, O. J., & Apkarian, R. P. (1993). Conventional and high resolution field emission scanning electron microscopy of vertebrate cerebellar parallel fiber-Purkinje spine synapses. Cellular Molecular Biology (Noisy-le-grand), 39(8), 863–873.
Castejón, O. J., & Villegas, G. M. (1964). Fine structure of synaptic contacts in stellate ganglion of squid. Journal of Ultrastructure Research, 10(5–6), 585–598.
Cerminara, N. L., Lang, E. J., Sillitoe, R. V., & Apps, R. (2015). Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nature Reviews Neuroscience, 16(2), 79–93.
Chazeau, A., & Giannone, G. (2016). Organization and dynamics of the actin cytoskeleton during dendritic spine morphological remodeling. Cellular and Molecular Life Science,. doi:10.1007/s00018-00016-02214-00011.
Chen, X., Levy, J. M., Hou, A., Winters, C., Azzam, R., Sousa, A. A., et al. (2015). PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proceedings of National Academy of Science USA, 112(50), E6983–E6992. doi:10.1073/pnas.1517045112.
Chen, W. R., Xiong, W. H., & Shepherd, G. M. (2000). Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron, 25(3), 625–633.
Chevy, Q., Heubl, M., Goutierre, M., Backer, S., Moutkine, I., Eugene, E., et al. (2015). KCC2 Gates Activity-Driven AMPA Receptor Traffic through Cofilin Phosphorylation. Journal of Neuroscience, 35(48), 15772–15786.
Chicurel, M. E., & Harris, K. M. (1992). Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. Journal of Comparative Neurology, 325(2), 169–182.
Chiu, C. Q., Lur, G., Morse, T. M., Carnevale, N. T., Ellis-Davies, G. C., & Higley, M. J. (2013). Compartmentalization of GABAergic inhibition by dendritic spines. Science, 340(6133), 759–762.
Chklovskii, D. B. (2004). Synaptic connectivity and neuronal morphology: Viewpoint two sides of the same coin. Neuron, 43(5), 609–617.
Chu, D. T. W., & Klymkowsky, M. W. (1989). The appearance of acetylated alpha-tubulin during early development and cellular-differentiation in Xenopus. Developmental Biology, 136(1), 104–117.
Clarke, D. J., & Dunnett, S. B. (1986). Ultrastructural organization of choline-acetyltransferase-immunoreactive fibres innervating the neocortex from embryonic ventral forebrain grafts. Journal of Comparative Neurology, 250(2), 192–205.
Clément, P. (1977). Ultrastructural research on rotifers. Archive Hydrobiol Beib Ergebn Limnol, 8, 270–297.
Coates, M. M. (2003). Visual ecology and functional morphology of cubozoa (cnidaria). Integrative and Comparative Biology, 43(4), 542–548.
Cobb, J. L. S., & Stubbs, T. R. (1982). The giant-neuron system in Ophiuroids. 3. The detailed connections of the circum-oral nerve ring. Cell and Tissue Research, 226(3), 675–687.
Cohen, A. I. (1973). Ultrastructural analysis of photoreceptors of squid and their synaptic connections. 3. Photoreceptor terminations in optic lobes. Journal of Comparative Neurology, 147(3), 399–425.
Colmers, W. F. (1977). Neuronal and synaptic organization in gravity receptor system of statocyst of Octopus-Vulgaris. Cell and Tissue Research, 185(4), 491–503.
Colonnier, M., & Guillery, R. W. (1964). Synaptic organization in the lateral geniculate nucleus of the monkey. Z Zellforsch Mikrosk Anat, 62, 333–355.
Cooney, J. R., Hurlburt, J. L., Selig, D. K., Harris, K. M., & Fiala, J. C. (2002). Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. Journal of Neuroscience, 22(6), 2215–2224.
Coss, R. G., Brandon, J. G., & Globus, A. (1980). Changes in morphology of dendritic spines on honeybee calycal interneurons associated with cumulative nursing and foraging experiences. Brain Research, 192(1), 49–59.
Coss, R. G., & Perkel, D. H. (1985). The function of dendritic spines: A review of theoretical issues. Behavioral and Neural Biology, 44(2), 151–185.
Dacheux, R. F., & Raviola, E. (1982). Horizontal cells in the retina of the rabbit. Journal of Neuroscience, 2(10), 1486–1493.
Darstein, M., Petralia, R. S., Swanson, G. T., Wenthold, R. J., & Heinemann, S. F. (2003). Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. Journal of Neuroscience, 23(22), 8013–8019.
De Robertis, E. D. P., & Bennett, H. S. (1955). Some features of the submicroscopic morphology of synapses in frog and earthworm. Journal of Biophysical and Biochemical Cytology,1(1)47–58 + 43 plates.
De Stefano, M. E., Luzzatto, A. C., & Mugnaini, E. (1993). Neuronal ultrastructure and somatostatin immunolocalization in the ciliary ganglion of chicken and quail. Journal of Neurocytology, 22(10), 868–892.
De Zeeuw, C. I., Gerrits, N. M., Voogd, J., Leonard, C. S., & Simpson, J. I. (1994). The rostral dorsal cap and ventrolateral outgrowth of the rabbit inferior olive receive a GABAergic input from dorsal group Y and the ventral dentate nucleus. Journal of Comparative Neurology, 341(3), 420–432.
Dehay, C., Douglas, R. J., Martin, K. A., & Nelson, C. (1991). Excitation by geniculocortical synapses is not ‘vetoed’ at the level of dendritic spines in cat visual cortex. Journal of Physiology, 440, 723–734.
Deller, T., Merten, T., Roth, S. U., Mundel, P., & Frotscher, M. (2000). Actin-associated protein synaptopodin in the rat hippocampal formation: Localization in the spine neck and close association with the spine apparatus of principal neurons. Journal of Comparative Neurology, 418(2), 164–181.
Deller, T., Orth, C. B., Del Turco, D., Vlachos, A., Burbach, G. J., Drakew, A., et al. (2007). A role for synaptopodin and the spine apparatus in hippocampal synaptic plasticity. Annals of Anatomy-Anatomischer Anzeiger, 189(1), 5–16.
Dent, E. W., Merriam, E. B., & Hu, X. (2011). The dynamic cytoskeleton: backbone of dendritic spine plasticity. Current Opinion in Neurobiology, 21(1), 175–181.
Descarries, L., Berube-Carriere, N., Riad, M., Bo, G. D., Mendez, J. A., & Trudeau, L. E. (2008). Glutamate in dopamine neurons: Synaptic versus diffuse transmission. Brain Research Reviews, 58(2), 290–302.
Descarries, L., Watkins, K. C., Garcia, S., & Beaudet, A. (1982). The serotonin neurons in nucleus raphe dorsalis of adult rat: A light and electron microscope radioautographic study. Journal of Comparative Neurology, 207(3), 239–254.
DeVries, S. H., Li, W., & Saszik, S. (2006). Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron, 50(5), 735–748.
DiGregorio, D. A., Nusser, Z., & Silver, R. A. (2002). Spillover of glutamate onto synaptic AMPA receptors enhances fast transmission at a cerebellar synapse. Neuron, 35(3), 521–533.
Dilly, P. N. (1969). Synapses in Cerebral Ganglion of Adult Ciona Intestinalis. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie, 93(1), 142–150.
Dilly, P. N. (1972). Structures of Tentacles of “Rhabdopleura-Compacta(Hemichordata) with special reference to neurociliary control. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie, 129(1), 20–39.
Dilly, P. N., Gray, E. G., & Young, J. Z. (1963). Electron microscopy of optic nerves and optic lobes of octopus and Eledone. Proceedings of the Royal Society Series B-Biological Sciences, 158(973), 446+.
Dilly, P. N., Welsch, U., & Storch, V. (1970). Structure of nerve fibre layer and neurocord in enteropneusts. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie, 103(1), 129–148.
Dryer, L., & Graziadei, P. P. C. (1996). Synaptology of the olfactory bulb of an elasmobranch fish Sphyrna tiburo. Anatomy and Embryology, 193(2), 101–114.
Dubin, M. W. (1970). The inner plexiform layer of the vertebrate retina: a quantitative and comparative electron microscopic analysis. Journal of Comparative Neurology, 140(4), 479–505.
Duman, C. H., & Duman, R. S. (2015). Spine synapse remodeling in the pathophysiology and treatment of depression. Neuroscience Letters, 601, 20–29.
Eid, T., Kovacs, I., Spencer, D. D., & de Lanerolle, N. C. (2002). Novel expression of AMPA-receptor subunit GluR1 on mossy cells and CA3 pyramidal neurons in the human epileptogenic hippocampus. European Journal of Neuroscience, 15(3), 517–527.
Ellwanger, K., Eich, A., & Nickel, M. (2007). GABA and glutamate specifically induce contractions in the sponge Tethya wilhelma. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology, 193(1), 1–11.
Ellwanger, K., & Nickel, M. (2006). Neuroactive substances specifically modulate rhythmic body contractions in the nerveless metazoon Tethya wilhelma (Demospongiae, Porifera). Frontiers in Zoology, 3, 7.
Emes, R. D., & Grant, S. G. (2012). Evolution of synapse complexity and diversity. Annual Review of Neuroscience, 35, 111–131.
Fahrenbach, W. H. (1979). Brain of the Horseshoe Crab (Limulus-Polyphemus).3. Cellular and synaptic organization of the Corpora Pedunculata. Tissue and Cell, 11(1), 163–200.
Famiglietti, E. V., & Peters, A. (1972). Synaptic glomerulus and intrinsic neuron in dorsal lateral geniculate nucleus of cat. Journal of Comparative Neurology, 144(3), 285–334.
Farris, S. M., Robinson, G. E., & Fahrbach, S. E. (2001). Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. Journal of Neuroscience, 21(16), 6395–6404.
Ferrero, E. (1973). Fine-structural analysis of statocyst in Turbellaria Acoela. Zoologica Scripta, 2(1), 5–16.
Fischbach, K. F., & Dittrich, A. P. M. (1989). The optic lobe of Drosophila-Melanogaster. 1. A golgi analysis of wild-Type structure. Cell and Tissue Research, 258(3), 441–475.
Fischer, F. P. (1992). Quantitative analysis of the innervation of the chicken basilar papilla. Hearing Research, 61(1–2), 167–178.
Fishelson, L. (1981). Observations on the moving colonies of the genus Tethya (Demospongia, Porifera).1. Behavior and cytology. Zoomorphology, 98(1), 89–99.
Fisher, S. K., & Boycott, B. B. (1974). Synaptic connections made by horizontal cells within outer plexiform layer of retina of cat and rabbit. Proceedings of the Royal Society Series B-Biological Sciences, 186(1085), 317.
Flood, P. R. (1966). A peculiar mode of muscular innervation in Amphioxus. Light and electron microscopic studies of the so-called ventral roots. Journal of Comparative Neurology, 126(2), 181–217.
Frambach, I., Rossler, W., Winkler, M., & Schurmann, F. W. (2004). F-actin at identified synapses in the mushroom body neuropil of the insect brain. Journal of Comparative Neurology, 475(3), 303–314.
Franc, J. M. (1978). Organization and function of ctenophore colloblasts: Ultrastructural-study. Biological Bulletin, 155(3), 527–541.
Freund, T. F., Powell, J. F., & Smith, A. D. (1984). Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience, 13(4), 1189–1215.
Frotscher, M., Seress, L., Schwerdtfeger, W. K., & Buhl, E. (1991). The mossy cells of the fascia-dentata: A comparative-study of their fine-structure and synaptic connections in rodents and primates. Journal of Comparative Neurology, 312(1), 145–163.
Frotscher, M., Studer, D., Graber, W., Chai, X., Nestel, S., & Zhao, S. (2014). Fine structure of synapses on dendritic spines. Frontiers in Neuroanatomy, 8, 94.
Fuchs, P. A. (2014). A ‘calcium capacitor’ shapes cholinergic inhibition of cochlear hair cells. Journal of Physiology, 592(Pt 16), 3393–3401.
Ganeshina, O., Berry, R. W., Petralia, R. S., Nicholson, D. A., & Geinisman, Y. (2004a). Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience, 125(3), 615–623.
Ganeshina, O., Berry, R. W., Petralia, R. S., Nicholson, D. A., & Geinisman, Y. (2004b). Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. Journal of Comparative Neurology, 468(1), 86–95.
Gardner, C. L., Jones, J. R., Baer, S. M., & Crook, S. M. (2015). Drift-diffusion simulation of the ephaptic effect in the triad synapse of the retina. Journal of Computational Neuroscience, 38(1), 129–142.
Garm, A., & Nilsson, D. E. (2014). Visual navigation in starfish: First evidence for the use of vision and eyes in starfish. Proceedings of the Royal Society B-Biological Sciences, 281(1777), 20133011. doi:10.1098/rspb.2013.3011.
Geiger, J. R., Lubke, J., Roth, A., Frotscher, M., & Jonas, P. (1997). Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron, 18(6), 1009–1023.
Geiger, J. R., Melcher, T., Koh, D. S., Sakmann, B., Seeburg, P. H., Jonas, P., & Monyer, H. (1995). Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron, 15(1), 193–204.
Goldman-Rakic, P. S., Leranth, C., Williams, S. M., Mons, N., & Geffard, M. (1989). Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proceedings of National Academy of Science USA, 86(22), 9015–9019.
Goosney, D. L., de Grado, M., & Finlay, B. B. (1999). Putting E. coli on a pedestal: a unique system to study signal transduction and the actin cytoskeleton. Trends in Cell Biology, 9(1), 11–14.
Gordon, D. P. (1974). Microarchitecture and function of lophophore in bryozoan Cryptosula-Pallasiana. Marine Biology, 27(2), 147–163.
Goto, J., & Mikoshiba, K. (2011). Inositol 1,4,5-trisphosphate receptor-mediated calcium release in Purkinje cells: from molecular mechanism to behavior. Cerebellum, 10(4), 820–833.
Granger, A. J., Mulder, N., Saunders, A., & Sabatini, B. L. (2016). Cotransmission of acetylcholine and GABA. Neuropharmacology, 100, 40–46.
Gray, E. G. (1959). Axo-somatic and axo-dendritic synapses of the cerebral cortex: An electron microscope study. Journal of Anatomy, 93, 420–433.
Gray, E. G. (1961). The granule cells, mossy synapses and Purkinje spine synapses of the cerebellum: Light and electron microscope observations. Journal of Anatomy, 95, 345–356.
Gray, E. G., & Guillery, R. W. (1963). A note on the dendritic spine apparatus. Journal of Anatomy, 97, 389–392.
Gray, G. C., Martin, V. J., & Satterlie, R. A. (2009). Ultrastructure of the retinal synapses in cubozoans. Biological Bulletin, 217(1), 35–49.
Gregory-Evans, K., Fariss, R. N., Possin, D. E., Gregory-Evans, C. Y., & Milam, A. H. (1998). Abnormal cone synapses in human cone-rod dystrophy. Ophthalmology, 105(12), 2306–2312.
Grigoryan, G., & Segal, M. (2016). Ryanodine-mediated conversion of STP to LTP is lacking in synaptopodin-deficient mice. Brain Structure and Function, 221(4), 2393–2397.
Groves, P. M., Linder, J. C., & Young, S. J. (1994). 5-hydroxydopamine-labeled dopaminergic axons: Three-dimensional reconstructions of axons, synapses and postsynaptic targets in rat neostriatum. Neuroscience, 58(3), 593–604.
Grunditz, A., Holbro, N., Tian, L., Zuo, Y., & Oertner, T. G. (2008). Spine neck plasticity controls postsynaptic calcium signals through electrical compartmentalization. Journal of Neuroscience, 28(50), 13457–13466.
Güldner, F. H. (1976). Synaptology of Rat Suprachiasmatic Nucleus. Cell and Tissue Research, 165(4), 509–544.
Gulledge, A. T., Carnevale, N. T., & Stuart, G. J. (2012). Electrical advantages of dendritic spines. PLoS One, 7(4), e36007. doi:10.1371/journal.pone.0036007.
Gulyas, A. I., Miettinen, R., Jacobowitz, D. M., & Freund, T. F. (1992). Calretinin is present in nonpyramidal cells of the rat hippocampus. 1. A new type of neuron specifically associated with the mossy fiber system. Neuroscience, 48(1), 1–27.
Günther, J., & Schürmann, F. W. (1973). Zur Feinstruktur des dorsalen riesenfasersystems im bauchmark des regenwurms. II. Synaptische beziehungen der proximalen riesenfaserkollateralen. Z Zellforsch, 139, 369–396.
Haas, M. A., Bell, D., Slender, A., Lana-Elola, E., Watson-Scales, S., Fisher, E. M. C., et al. (2013). Alterations to dendritic spine morphology, but not dendrite patterning, of cortical projection neurons in Tc1 and Ts1Rhr mouse models of down syndrome. PLoS One, 8(10), e78561.
Hafner, G. S. (1974). The ultrastructure of retinula cell endings in the compound eye of the crayfish. Journal of Neurocytology, 3(3), 295–311.
Hall, D. H., & Russell, R. L. (1991). The posterior nervous-system of the nematode Caenorhabditis-Elegans: Serial reconstruction of identified neurons and complete pattern of synaptic-interactions. Journal of Neuroscience, 11(1), 1–22.
Halpain, S. (2000). Actin and the agile spine: how and why do dendritic spines dance? Trends in Neurosciences, 23(4), 141–146.
Halton, D. W., & Gustafsson, M. K. S. (1996). Functional morphology of the platyhelminth nervous system. Parasitology, 113, S47–S72.
Hama, K. (1961). Some observations on fine structure of giant fibers of crayfishes (Cambarus Virilus and Cambarus Clarkii) with special reference to submicroscopic organization of synapses. Anatomical Record, 141(4), 275–293.
Hama, K. (1962). Some Observations on the fine structure of the giant synapse in the stellate ganglion of the squid, Doryteuphis-Bleekeri. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie, 56(4), 437–444.
Hamill, G. S., & Lenn, N. J. (1983). Synaptic plasticity within the interpeduncular nucleus after unilateral lesions of the habenula in neonatal rats. Journal of Neuroscience, 3(11), 2128–2145.
Hamilton, D. W. (1968). The calyceal synapse of type I vestibular hair cells. Journal of Ultrastructure Research, 23(1), 98–114.
Hamlyn, L. H. (1962). Fine Structure of Mossy Fibre Endings in Hippocampus of Rabbit. Journal of Anatomy, 96(Jan), 112–120 + 116 plates.
Hamori, J., & Horridge, G. A. (1966). Lobster optic lamina. 2. Types of synapse. Journal of Cell Science, 1(2), 257–270.
Hamori, J., Pasik, T., Pasik, P., & Szentagothai, J. (1974). Triadic synaptic arrangements and their possible significance in the lateral geniculate nucleus of the monkey. Brain Research, 80(3), 379–393.
Harnett, M. T., Makara, J. K., Spruston, N., Kath, W. L., & Magee, J. C. (2012). Synaptic amplification by dendritic spines enhances input cooperativity. Nature, 491(7425), 599+.
Harris, K. M., Jensen, F. E., & Tsao, B. (1992). Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. Journal of Neuroscience, 12(7), 2685–2705.
Harris, K. M., & Kater, S. B. (1994). Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annual Review of Neuroscience, 17, 341–371.
Harris, K. M., & Weinberg, R. J. (2012). Ultrastructure of synapses in the mammalian brain. Cold Spring Harbor Perspectives in Biology, 4(5), a005587. doi:10.1101/cshperspect.a005587.
Hartline, D. K., & Colman, D. R. (2007). Rapid conduction and the evolution of giant axons and myelinated fibers. Current Biology, 17(1), R29–R35.
Hausen, K., Wolburgbuchholz, K., & Ribi, W. A. (1980). The synaptic organization of visual interneurons in the lobula complex of flies: A light and electron microscopical study using silver-intensified Cobalt-Impregnations. Cell and Tissue Research, 208(3), 371–387.
Haverkamp, S., Grunert, U., & Wassle, H. (2000). The cone pedicle, a complex synapse in the retina. Neuron, 27(1), 85–95.
Heimberg, A. M., Cowper-Sallari, R., Semon, M., Donoghue, P. C. J., & Peterson, K. J. (2010). microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proceedings of the National Academy of Sciences of the United States of America, 107(45), 19379–19383.
Henselmans, J. M. L., & Wouterlood, F. G. (1994). Light and electron-microscopic characterization of cholinergic and dopaminergic structures in the striatal complex and the dorsal ventricular ridge of the lizard Gekko-Gecko. Journal of Comparative Neurology, 345(1), 69–83.
Henze, D. A., Urban, N. N., & Barrionuevo, G. (2000). The multifarious hippocampal mossy fiber pathway: A review. Neuroscience, 98(3), 407–427.
Henze, D. A., Wittner, L., & Buzsaki, G. (2002). Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nature Neuroscience, 5(8), 790–795.
Hering, H., & Sheng, M. (2001). Dendritic spines: Structure, dynamics and regulation. Nature Reviews Neuroscience, 2(12), 880–888.
Hernandez-Nicaise, M. L. (1973). The nervous system of ctenophores. III. Ultrastructure of synapses. Journal of Neurocytology, 2(3), 249–263.
Hersch, S. M., Ciliax, B. J., Gutekunst, C. A., Rees, H. D., Heilman, C. J., Yung, K. K., et al. (1995). Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. Journal of Neuroscience, 15(7 Pt 2), 5222–5237.
Hirokawa, N. (1978). The ultrastructure of the basilar papilla of the chick. Journal of Comparative Neurology, 181(2), 361–374.
Hnasko, T. S., & Edwards, R. H. (2012). Neurotransmitter corelease: Mechanism and physiological role. Annual Review of Physiology, 74, 225–243.
Holmberg, K. (1970). Hagfish retina: Fine structure of retinal cells in Myxine-Glutinosa, L, with special reference to receptor and epithelial cells. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie, 111(4), 519–538.
Holmberg, K. (1971). Hagfish retina: Electron microscopic study comparing receptor and epithelial cells in Pacific Hagfish, Polistotrema-Stouti, with those in Atlantic Hagfish, Myxine-Glutinosa. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie, 121(2), 249–269.
Holmberg, K., & Ohman, P. (1976). Fine-structure of retinal synaptic organelles in lamprey and hagfish photoreceptors. Vision Research, 16(3), 237–239.
Holtmann, M., & Thurm, U. (2001). Mono- and oligo-vesicular synapses and their connectivity in a Cnidarian sensory epithelium (Coryne tubulosa). Journal of Comparative Neurology, 432(4), 537–549.
Hoogenraad, C. C., & Akhmanova, A. (2010). Dendritic spine plasticity: New regulatory roles of dynamic microtubules. Neuroscientist, 16(6), 650–661.
Hoogenraad, C. C., & Bradke, F. (2009). Control of neuronal polarity and plasticity–a renaissance for microtubules? Trends in Cell Biology, 19(12), 669–676.
Horak, M., Petralia, R. S., Kaniakova, M., & Sans, N. (2014). ER to synapse trafficking of NMDA receptors. Frontiers in Cell Neuroscience, 8, 394.
Horn, G., Bradley, P., & Mccabe, B. J. (1985). Changes in the structure of synapses associated with learning. Journal of Neuroscience, 5(12), 3161–3168.
Houser, C. R., Crawford, G. D., Salvaterra, P. M., & Vaughn, J. E. (1985). Immunocytochemical localization of choline acetyltransferase in rat cerebral cortex: A study of cholinergic neurons and synapses. Journal of Comparative Neurology, 234(1), 17–34.
Hu, H., Gan, J., & Jonas, P. (2014). Interneurons. Fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science, 345(6196), 1255263.
Huganir, R. L., & Nicoll, R. A. (2013). AMPARs and synaptic plasticity: The last 25 years. Neuron, 80(3), 704–717.
Ichikawa, M. (1976). Fine-structure of olfactory-bulb in goldfish Carassius-Auratus. Brain Research, 115(1), 53–56.
Jahr, C. E., & Nicoll, R. A. (1982). An Intracellular analysis of dendrodendritic inhibition in the turtle invitro Olfactory-Bulb. Journal of Physiology-London, 326(May), 213–234.
Jedlicka, P., Vlachos, A., Schwarzacher, S. W., & Deller, T. (2008). A role for the spine apparatus in LTP and spatial learning. Behavioural Brain Research, 192(1), 12–19.
Johnston, D., & Amaral, D. G. (2004). Hippocampus. In G. M. Shepherd (Ed.), The synaptic organization of the brain (5th ed., pp. 455–498). New York: Oxford University Press.
Jones, E. G., & Powell, T. P. (1969). Morphological variations in the dendritic spines of the neocortex. Journal of Cell Science, 5(2), 509–529.
Jones, E. G., & Powell, T. P. (1970). Electron microscopy of the somatic sensory cortex of the cat. I. Cell types and synaptic organization. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 257(812), 1–11.
Jorgensen, E. M. (2014). Animal evolution: looking for the first nervous system. Current Biology, 24(14), R655–R658.
Kaifu, K., Akamatsu, T., & Segawa, S. (2008). Underwater sound detection by cephalopod statocyst. Fisheries Science, 74(4), 781–786.
Kapadia, S. E., de Lanerolle, N. C., & LaMotte, C. C. (1985). Immunocytochemical and electron microscopic study of serotonin neuronal organization in the dorsal raphe nucleus of the monkey. Neuroscience, 15(3), 729–746.
Kasa, P., Dobo, E., & Wolff, J. R. (1991). Cholinergic innervation of the mouse superior cervical ganglion: light- and electron-microscopic immunocytochemistry for choline acetyltransferase. Cell and Tissue Research, 265(1), 151–158.
Keenan, C. L., Coss, R., & Koopowitz, H. (1981). Cytoarchitecture of primitive brains: Golgi studies in flatworms. Journal of Comparative Neurology, 195(4), 697–716.
Kelava, I., Rentzsch, F., & Technau, U. (2015). Evolution of eumetazoan nervous systems: Insights from cnidarians. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 370(1684), 20150065. doi:10.1098/rstb.2015.0065.
Kelly, D. E., & Smith, S. W. (1964). Fine structure of the pineal organs of the adult frog, Rana Pipiens. Journal of Cell Biology, 22, 653–674.
Knott, G. W., Quairiaux, C., Genoud, C., & Welker, E. (2002). Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron, 34(2), 265–273.
Koizumi, O., Sato, N., & Goto, C. (2004). Chemical anatomy of hydra nervous system using antibodies against hydra neuropeptides: A review. Hydrobiologia, 530, 41–47.
Kolb, H. (1977). The organization of the outer plexiform layer in the retina of the cat: Electron microscopic observations. Journal of Neurocytology, 6(2), 131–153.
Kopec, C. D., Real, E., Kessels, H. W., & Malinow, R. (2007). GluR1 links structural and functional plasticity at excitatory synapses. Journal of Neuroscience, 27(50), 13706–13718.
Korkotian, E., Frotscher, M., & Segal, M. (2014). Synaptopodin regulates spine plasticity: Mediation by calcium stores. Journal of Neuroscience, 34(35), 11641–11651.
Korkotian, E., & Segal, M. (2007). Morphological constraints on calcium dependent glutamate receptor trafficking into individual dendritic spine. Cell Calcium, 42(1), 41–57.
Kotsyuba, E. P., & Kotsyuba, A. E. (2002). Ultrastructural characteristics of interneuronal connections of the central nervous system of bivalve molluscs. Journal of Evolutionary Biochemistry and Physiology, 38(3), 330–335.
Kramer, R. H., & Davenport, C. M. (2015). Lateral inhibition in the vertebrate retina: The case of the missing neurotransmitter. PLoS Biology, 13(12), e1002322.
Kubota, Y., Hatada, S., Kondo, S., Karube, F., & Kawaguchi, Y. (2007). Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. Journal of Neuroscience, 27(5), 1139–1150.
Lacalli, T. C. (2002). The dorsal compartment locomotory control system in amphioxus larvae. Journal of Morphology, 252(3), 227–237.
Lacalli, T. C., & Kelly, S. J. (2003). Sensory pathways in amphioxus larvae II. Dorsal tracts and translumenal cells. Acta Zoologica, 84(1), 1–13.
Ladepeche, L., Dupuis, J. P., Bouchet, D., Doudnikoff, E., Yang, L., Campagne, Y., et al. (2013). Single-molecule imaging of the functional crosstalk between surface NMDA and dopamine D1 receptors. Proc Natl Acad Sci U S A, 110(44), 18005–18010.
Lai, K. O., & Ip, N. Y. (2013). Structural plasticity of dendritic spines: The underlying mechanisms and its dysregulation in brain disorders. Biochimica Et Biophysica Acta-Molecular Basis of Disease, 1832(12), 2257–2263.
Lamb, T. D., Collin, S. P., & Pugh, E. N. (2007). Evolution of the vertebrate eye: Opsins, photoreceptors, retina and eye cup. Nature Reviews Neuroscience, 8(12), 960–975.
Lamb, T. D., Pugh, E. N, Jr, & Collin, S. P. (2008). The origin of the vertebrate eye. Evolution Eduation and Outreach, 1, 415–426.
Lannoo, M. J., & Hawkes, R. (1997). A search for primitive Purkinje cells: Zebrin II expression in sea lampreys (Petromyzon marinus). Neuroscience Letters, 237(1), 53–55.
Laughlin, S. B. (1973). Neural integration in first optic neuropil of dragonflies. 1. signal amplification in Dark-Adapted second-order Neurons. Journal of Comparative Physiology, 84(4), 335–355.
Lawrence, J. J., & McBain, C. J. (2003). Interneuron diversity series: Containing the detonation–feedforward inhibition in the CA3 hippocampus. Trends in Neurosciences, 26(11), 631–640.
Lehmann, T., Hess, M., Wanner, G., & Melzer, R. R. (2014). Dissecting a neuron network: FIB-SEM-based 3D-reconstruction of the visual neuropils in the sea spider Achelia langi (Dohrn, 1881) (Pycnogonida). BMC Biology, 12, 59.
Leiss, F., Groh, C., Butcher, N. J., Meinertzhagen, I. A., & Tavosanis, G. (2009a). Synaptic organization in the adult Drosophila mushroom body calyx. Journal of Comparative Neurology, 517(6), 808–824.
Leiss, F., Koper, E., Hein, I., Fouquet, W., Lindner, J., Sigrist, S., & Tavosanis, G. (2009b). Characterization of dendritic spines in the Drosophila central nervous system. Dev Neurobiol, 69(4), 221–234.
Lenn, N. J., Leranth, C., & Zaborszky, L. (1985). Choline acetyltransferase immunoreactivity is localized to four types of synapses in the rat interpeduncular nucleus. Journal of Neurocytology, 14(6), 909–919.
Leys, S. P. (2015). Elements of a ‘nervous system’ in sponges. Journal of Experimental Biology, 218(Pt 4), 581–591.
Li, Y., Hough, C. J., Frederickson, C. J., & Sarvey, J. M. (2001). Induction of mossy fiber →Ca3 long-term potentiation requires translocation of synaptically released Zn2+. Journal of Neuroscience, 21(20), 8015–8025.
Lichnerova, K., Kaniakova, M., Park, S. P., Skrenkova, K., Wang, Y. X., Petralia, R. S., et al. (2015). Two N-glycosylation sites in the GluN1 subunit are essential for releasing N-methyl-d-aspartate (NMDA) receptors from the Endoplasmic reticulum. Journal of Biological Chemistry, 290(30), 18379–18390.
Lieberman, A. R. (1971). Microtubule-associated smooth endoplasmic reticulum in the frog’s brain. Z Zellforsch Mikrosk Anat, 116(4), 564–577.
Llinás, R. R., Walton, K. D., & Lang, E. J. (2004). Cerebellum. In G. M. Shepherd (Ed.), The synaptic organization of the brain (5th ed., pp. 271–309). New York: Oxford University Press.
Lopez-Garcia, C., & Martinez-Guijarro, F. J. (1988). Neurons in the medial cortex give rise to Timm-positive boutons in the cerebral cortex of lizards. Brain Research, 463(2), 205–217.
Mackie, G. O., Lawn, I. D., & Dececcatty, M. P. (1983). Studies on Hexactinellid Sponges. 2. Excitability, conduction and coordination of responses in Rhabdocalyptus-Dawsoni (Lambe, 1873). Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 301(1107), 401–418.
Maggio, N., & Vlachos, A. (2014). Synaptic plasticity at the interface of health and disease: New insights on the role of endoplasmic reticulum intracellular calcium stores. Neuroscience, 281C, 135–146.
Mäntylä, K., Reuter, M., Halton, D. W., Maule, A. G., Brennan, G. P., Shaw, C., & Gustafsson, M. K. S. (1998). The nervous system of Procerodes littoralis (Maricola, Tricladida). An ultrastructural and immunoelectron microscopical study. Acta Zoologica, 79(1), 1–8.
Marlow, H. Q., Srivastava, M., Matus, D. Q., Rokhsar, D., & Martindale, M. Q. (2009). Anatomy and development of the nervous system of Nematostella vectensis, an Anthozoan Cnidarian. Developmental Neurobiology, 69(4), 235–254.
Martin, V. J. (2002). Photoreceptors of cnidarians. Canadian Journal of Zoology, 80(10), 1703–1722.
Martin, V. J. (2004). Photoreceptors of cubozoan jellyfish. Hydrobiologia, 530, 135–144.
Martinez Guijarro, F. J., Berbel, P. J., Molowny, A., & Lopez Garcia, C. (1984). Apical dendritic spines and axonic terminals in the bipyramidal neurons of the dorsomedial cortex of lizards (Lacerta). Anatomy and Embryology (Berl), 170(3), 321–326.
Mashanov, V. S., Zueva, O. R., & Heinzeller, T. (2008). Regeneration of the radial nerve cord in a holothurian: A promising new model system for studying post-traumatic recovery in the adult nervous system. Tissue and Cell, 40(5), 351–372.
Mashanov, V. S., Zueva, O. R., Heinzeller, T., & Dolmatov, I. Y. (2006). Ultrastructure of the circumoral nerve ring and the radial nerve cords in holothurians (Echinodermata). Zoomorphology, 125(1), 27–38.
Meek, J., & Nieuwenhuys, R. (1991). Palisade pattern of mormyrid Purkinje cells: a correlated light and electron microscopic study. Journal of Comparative Neurology, 306(1), 156–192.
Megias, M., Emri, Z., Freund, T. F., & Gulyas, A. I. (2001). Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience, 102(3), 527–540.
Meinertzhagen, I. A., & O’Neil, S. D. (1991). Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. Journal of Comparative Neurology, 305(2), 232–263.
Miglietta, M. P., Della Tommasa, L., Denitto, F., Gravili, C., Pagliara, P., Bouillon, J., & Boero, F. (2000). Approaches to the ethology of hydroids and medusae (Cnidaria, Hydrozoa). Scientia Marina, 64, 63–71.
Mikami, Y., Yoshida, T., Matsuda, N., & Mishina, M. (2004). Expression of zebrafish glutamate receptor delta2 in neurons with cerebellum-like wiring. Biochem Biophys Res Commun, 322(1), 168–176.
Milhaud, M., & Pappas, G. D. (1966). Postsynaptic bodies in Habenula and interpeduncular Nuclei of cat. Journal of Cell Biology, 30(2), 437–441.
Min, M. Y., Rusakov, D. A., & Kullmann, D. M. (1998). Activation of AMPA, kainate, and metabotropic receptors at hippocampal mossy fiber synapses: Role of glutamate diffusion. Neuron, 21(3), 561–570.
Mizuno, N., & Nakamura, Y. (1972). An electron microscope study of the locus coeruleus in the rabbit, with special reference to direct hypothalamic and mesencephalic projections. Archives of Histology Japan, 34(5), 433–448.
Moraczewski, J., Czubaj, A., & Bakowska, J. (1977). Organization and ultrastructure of nervous-system in Catenulida (Turbellaria). Zoomorphologie, 87(1), 87–95.
Morita, M., & Best, J. B. (1966). Electron microscopic studies of planaria. 3. Some observations on fine structure of planarian nervous tissue. Journal of Experimental Zoology, 161(3), 391–412.
Moroz, L. L., Kocot, K. M., Citarella, M. R., Dosung, S., Norekian, T. P., Povolotskaya, I. S., et al. (2014). The ctenophore genome and the evolutionary origins of neural systems. Nature, 510(7503), 109–114.
Moroz, L. L., & Kohn, A. B. (2016). Independent origins of neurons and synapses: Insights from ctenophores. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 371(1685), 20150041.
Moshkov, D. A., Shtanchaev, R. S., Mikheeva, I. B., Bezgina, E. N., Kokanova, N. A., Mikhailova, G. Z., et al. (2013). Visual input controls the functional activity of goldfish Mauthner neuron through the reciprocal synaptic mechanism. Journal of Integrative Neuroscience, 12(1), 17–34.
Mugnaini, E. (1972). The histology and cytology of the cerebellar cortex. In O. Larsell & J. Jansen (Eds.), The comparative anatomy and histology of the cerebellum. The human cerebellum, cerebellar connections, and cerebellar cortex (pp. 201–264 + 267 plates). Minneapolis: The University of Minnesota Press.
Muller, J. F., Mascagni, F., Zaric, V., Mott, D. D., & McDonald, A. J. (2016). Localization of the M2 muscarinic cholinergic receptor in dendrites, cholinergic terminals, and noncholinergic terminals in the rat basolateral amygdala: An ultrastructural analysis. Journal of Comparative Neurology,. doi:10.1002/cne.23959.
Muller, K. J., & McMahan, U. J. (1976). The shapes of sensory and motor neurones and the distribution of their synapses in ganglia of the leech: a study using intracellular injection of horseradish peroxidase. Proceedings of the Royal Society of London. Series B: Biological Sciences, 194(1117), 481–499.
Mundel, P., Heid, H. W., Mundel, T. M., Kruger, M., Reiser, J., & Kriz, W. (1997). Synaptopodin: An actin-associated protein in telencephalic dendrites and renal podocytes. Journal of Cell Biology, 139(1), 193–204.
Murray, M., Zimmer, J., & Raisman, G. (1979). Quantitative electron-microscopic evidence for Re-innervation in the adult-Rat interpeduncular nucleus after lesions of the fasciculus retroflexus. Journal Of Comparative Neurology, 187(2), 447–468.
Nag, T. C., & Wadhwa, S. (2012). Ultrastructure of the human retina in aging and various pathological states. Micron, 43(7), 759–781.
Nakamura, P. A., & Cramer, K. S. (2011). Formation and maturation of the calyx of Held. Hearing Research, 276(1–2), 70–78.
Nässel, D. R. (1977). Types and arrangements of neurons in crayfish optic lamina. Cell and Tissue Research, 179(1), 45–75.
Nässel, D. R., & Strausfeld, N. J. (1982). A pair of descending neurons with dendrites in the optic lobes projecting directly to thoracic ganglia of dipterous insects. Cell and Tissue Research, 226(2), 355–362.
Nässel, D. R., & Waterman, T. H. (1977). Golgi em evidence for visual formation channeling in crayfish lamina ganglionaris. Brain Research, 130(3), 556–563.
Nek, N., Schwegler, H., Crusio, W. E., & Frotscher, M. (1993). Are the fine-structural characteristics of mouse hippocampal mossy fiber synapses determined by the density of mossy fiber axons. Neuroscience Letters, 158(1), 75–78.
Nelson, R., Lutzow, A. V., Kolb, H., & Gouras, P. (1975). Horizontal cells in cat retina with independent dendritic systems. Science, 189(4197), 137–139.
Newpher, T. M., & Ehlers, M. D. (2009). Spine microdomains for postsynaptic signaling and plasticity. Trends in Cell Biology, 19(5), 218–227.
Nickel, M. (2004). Kinetics and rhythm of body contractions in the sponge Tethya wilhelma (Porifera: Demospongiae). Journal of Experimental Biology, 207(26), 4515–4524.
Nicoll, R. A., & Schmitz, D. (2005). Synaptic plasticity at hippocampal mossy fibre synapses. Nature Reviews Neuroscience, 6(11), 863–876.
Nilsson, D. E., Gislen, L., Coates, M. M., Skogh, C., & Garm, A. (2005). Advanced optics in a jellyfish eye. Nature, 435(7039), 201–205.
Nitsch, C., & Rinne, U. (1981). Large Dense-Core vesicle exocytosis and membrane recycling in the mossy fiber synapses of the rabbit hippocampus during epileptiform seizures. Journal of Neurocytology, 10(2), 201–219.
Nomokonova, L. M., & Ozirskaya, E. V. (1984). Morphological study of middle and posterior hypothalamic projections to forebrain in the pond turtle. Neuroscience and Behavioral Physiology, 14(4), 282–290.
Oksche, A., & von Harnack, M. (1963). Elektronenmikroskopische Untersuchungen Am Stirnorgan Von Anuren - (Zur Frage Der Lichtrezeptoren). Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie, 59(2), 239–288.
Okubo, Y., Suzuki, J., Kanemaru, K., Nakamura, N., Shibata, T., & Iino, M. (2015). Visualization of Ca2+ filling mechanisms upon synaptic inputs in the endoplasmic reticulum of cerebellar Purkinje cells. Journal of Neuroscience, 35(48), 15837–15846.
Oliver, D., Brinkmann, M., Sieger, T., & Thurm, U. (2008). Hydrozoan nematocytes send and receive synaptic signals induced by mechanochemical stimuli. Journal of Experimental Biology, 211(17), 2876–2888.
Orth, C. B., Schultz, C., Muller, C. M., Frotscher, M., & Deller, T. (2007). Loss of the cisternal organelle in the axon initial segment of cortical neurons in synaptopodin-deficient mice. Journal of Comparative Neurology, 504(5), 441–449.
Palay, S. L., & Chan-Palay, V. (1974). Cerebellar cortex. cytology and organization. New York: Springer.
Palay, S. L., Sotelo, C., Peters, A., & Orkand, P. M. (1968). Axon hillock and initial segment. Journal of Cell Biology, 38(1), 193–201.
Paoletti, P., Bellone, C., & Zhou, Q. (2013). NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nature Reviews Neuroscience, 14(6), 383–400.
Papadopoulos, G. C., Parnavelas, J. G., & Buijs, R. M. (1989). Light and electron microscopic immunocytochemical analysis of the noradrenaline innervation of the rat visual cortex. Journal of Neurocytology, 18(1), 1–10.
Pardos, F., Roldan, C., Benito, J., & Emig, C. C. (1991). Fine-Structure of the tentacles of Phoronis-Australis Haswell (Phoronida, Lophophorata). Acta Zoologica, 72(2), 81–90.
Parkefelt, L., Skogh, C., Nilsson, D. E., & Ekstrom, P. (2005). Bilateral symmetric organization of neural elements in the visual system of a coelenterate, Tripedalia cystophora (Cubozoa). Journal of Comparative Neurology, 492(3), 251–262.
Pasch, E., Muenz, T. S., & Rossler, W. (2011). CaMKII is differentially localized in synaptic regions of Kenyon cells within the mushroom bodies of the honeybee brain. Journal of Comparative Neurology, 519(18), 3700–3712.
Pasik, T., & Pasik, P. (1982). Serotoninergic afferents in the monkey neostriatum. Acta biologica Academiae Scientiarum Hungaricae, 33(2–3), 277–288.
Pavans de Ceccatty, M. (1966). Ultrastructures et rapports des cellules mesenchymateuses de type nerveux de l’eponge Tethya lyncurium Link. Annals Science Nature Zoology, 8, 577–614.
Peña-Contreras, Z., Mendoza-Briceno, R. V., Miranda-Contreras, L., & Palacios-Pru, E. L. (2007). Synaptic dimorphism in Onychophoran cephalic ganglia. Revista de Biologia Tropical, 55(1), 261–267.
Peters, B. H., & Campbell, A. C. (1987). Morphology of the nervous and muscular systems in the heads of pedicellariae from the Sea-Urchin Echinus-Esculentus L. Journal of Morphology, 193(1), 35–51.
Peters, A., & Kaiserman-Abramof, I. R. (1970). The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. American Journal of Anatomy, 127(4), 321–355.
Peters, A., Palay, S. L., & Webster, Hd. (1991). The fine structure of the nervous system. Neurons and their supporting cells (3rd ed.). New York: Oxford.
Petralia, R. S. (2012). Distribution of extrasynaptic NMDA receptors on neurons. Scientific World Journal. doi:10.1100/2012/267120.
Petralia, R. S., Mattson, M. P., & Yao, P. J. (2014). Communication breakdown: The impact of ageing on synapse structure. Ageing Research and Reviews, 14, 31–42.
Petralia, R. S., Wang, Y. X., Hua, F., Yi, Z., Zhou, A., Ge, L., et al. (2010). Organization of NMDA receptors at extrasynaptic locations. Neuroscience, 167(1), 68–87.
Petralia, R. S., Wang, Y. X., Mattson, M. P., & Yao, P. J. (2011). Sonic hedgehog distribution within mature hippocampal neurons. Communicative and Integrative Biology, 4(6), 775–777.
Petralia, R. S., Wang, Y. X., Mattson, M. P., & Yao, P. J. (2015). Structure, distribution, and function of neuronal/synaptic spinules and related invaginating projections. Neuromolecular Medicine, 17(3), 211–240.
Petralia, R. S., Wang, Y. X., Sans, N., Worley, P. F., Hammer, J. A, 3rd, & Wenthold, R. J. (2001). Glutamate receptor targeting in the postsynaptic spine involves mechanisms that are independent of myosin Va. European Journal of Neuroscience, 13(9), 1722–1732.
Petralia, R. S., Wang, Y. X., & Wenthold, R. J. (2002). NMDA receptors and PSD-95 are found in attachment plaques in cerebellar granular layer glomeruli. European Journal of Neuroscience, 15(3), 583–587.
Petralia, R. S., Wang, Y. X., Zhao, H. M., & Wenthold, R. J. (1996). Ionotropic and metabotropic glutamate receptors show unique postsynaptic, presynaptic, and glial localizations in the dorsal cochlear nucleus. Journal of Comparative Neurology, 372(3), 356–383.
Petralia, R. S., & Wenthold, R. J. (1992). Light and electron immunocytochemical localization of ampa-selective glutamate receptors in the rat-brain. Journal of Comparative Neurology, 318(3), 329–354.
Petralia, R. S., & Wenthold, R. J. (1998). Glutamate receptor antibodies. Production and Immunocytochemistry. In M. A. Ariano (Ed.), Receptor localization: Laboratory methods and procedures (pp. 46–74). New York: Wiley.
Petralia, R. S., & Wenthold, R. J. (1999). Immunocytochemistry of NMDA receptors. Methods in Molecular Biology, 128, 73–92.
Pisani, D., Pett, W., Dohrmann, M., Feuda, R., Rota-Stabelli, O., Philippe, H., et al. (2015). Genomic data do not support comb jellies as the sister group to all other animals. Proceedings of National Academy of Science USA, 112(50), 15402–15407.
Price, J. L., & Powell, T. P. S. (1970a). An electron-microscopic study of termination of afferent fibres to olfactory bulb from cerebral hemisphere. Journal of Cell Science, 7(1), 157–187.
Price, J. L., & Powell, T. P. S. (1970b). Synaptology of granule cells of olfactory bulb. Journal of Cell Science, 7(1), 125–155.
Prince, F. P., & Jones-Witters, P. H. (1974). The ultrstructure of the medical preoptic area of the rat. Cell and Tissue Research, 153(4), 517–530.
Purves, D., & McMahan, U. J. (1972). The distribution of synapses on a physiologically identified motor neuron in the central nervous system of the leech. An electron microscope study after the injection of the fluorescent dye procion yellow. Journal of Cell Biology, 55(1), 205–220.
Racz, B., & Weinberg, R. J. (2013). Microdomains in forebrain spines: An ultrastructural perspective. Molecular Neurobiology, 47(1), 77–89.
Raikova, O. I., Reuter, M., Jondelius, U., & Gustafsson, M. K. S. (2000). An immunocytochemical and ultrastructural study of the nervous and muscular systems of Xenoturbella westbladi (Bilateria inc. sed.). Zoomorphology, 120(2), 107–118.
Rall, W. (1974). Dendritic spines, synaptic potency and neuronal plasticity. In C. D. Woody, K. A. Brown, T. J. Crow, & J. D. Knispel (Eds.), Cellular mechanisms subserving changes in neuronal activity (pp. 13–21). Los Angeles, CA: Brain Information Service.
Rall, W., & Rinzel, J. (1971). Dendritic spine function and synaptic attenuation calculations. Society for Neuroscience Abstracts, 1, 64.
Rall, W., & Rinzel, J. (1973). Branch input resistance and steady attenuation for input to one branch of a dendritic neuron model. Biophysical Journal, 13(7), 648–688.
Rall, W., & Shepherd, G. M. (1968). Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. Journal of Neurophysiology, 31(6), 884–915.
Rall, W., Shepherd, G. M., Reese, T. S., & Brightma, M. W. (1966). Dendrodendritic synaptic pathway for inhibition in olfactory bulb. Experimental Neurology, 14(1), 44–56.
Rehkämper, G., & Welsch, U. (1985). On the fine-structure of the cerebral ganglion of sagitta (Chaetognatha). Zoomorphology, 105(2), 83–89.
Reuter, M. (1981). The nervous-system of microstomum-lineare (Turbellaria, Macrostomida).2. The ultrastructure of synapses and neurosecretory release sites. Cell and Tissue Research, 218(2), 375–387.
Reuter, M., & Palmberg, I. (1990). Synaptic and nonsynaptic release in Stenostomum-Leucops. A study of the nervous-system and sensory receptors. Early Brain, 5, 121–136.
Rodriguez, A., Ehlenberger, D. B., Dickstein, D. L., Hof, P. R., & Wearne, S. L. (2008). Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One, 3(4), e1997.
Rollenhagen, A., Satzler, K., Rodriguez, E. P., Jonas, P., Frotscher, M., & Lubke, J. H. R. (2007). Structural determinants of transmission at large hippocampal mossy fiber Synapses. Journal of Neuroscience, 27(39), 10434–10444.
Root, D. H., Mejias-Aponte, C. A., Zhang, S., Wang, H. L., Hoffman, A. F., Lupica, C. R., & Morales, M. (2014). Single rodent mesohabenular axons release glutamate and GABA. Nature Neuroscience, 17(11), 1543–1551.
Rosenbluth, J. (1962). Subsurface cisterns and their relationship to the neuronal plasma membrane. Journal of Cell Biology, 13, 405–421.
Rosenbluth, J. (1965). Ultrastructure of somatic muscle cells in Ascaris lumbricoides. II. Intermuscular junctions, neuromuscular junctions, and glycogen stores. Journal of Cell Biology, 26(2), 579–591.
Ryan, J. F., & Chiodin, M. (2015). Where is my mind? How sponges and placozoans may have lost neural cell types. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 370(1684), 20150059. doi:10.1098/rstb.2015.0059.
Ryugo, D. K., Montey, K. L., Wright, A. L., Bennett, M. L., & Pongstaporn, T. (2006). Postnatal development of a large auditory nerve terminal: the endbulb of held in cats. Hearing Research, 216–217, 100–115.
Sala, C., & Segal, M. (2014). Dendritic spines: The locus of structural and functional plasticity. Physiological Reviews, 94(1), 141–188.
Sätzler, K., Söhl, L. F., Bollmann, J. H., Borst, J. G. G., Frotscher, M., Sakmann, B., & Lübke, J. H. R. (2002). Three-dimensional reconstruction of a calyx of held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. Journal of Neuroscience, 22(24), 10567–10579.
Schaeffer, S. F., & Raviola, E. (1976). Ultrastructural analysis of functional changes in the synaptic endings of turtle cone cells. Cold Spring Harbor Symposia on Quantitative Biology, 40, 521–528.
Schmidt, B., Marrone, D. F., & Markus, E. J. (2012). Disambiguating the similar: the dentate gyrus and pattern separation. Behavioural Brain Research, 226(1), 56–65.
Schürmann, F. W. (1978). Note on structure of synapses in ventral nerve cord of onychophoran Peripatoides-Leuckarti. Cell and Tissue Research, 186(3), 527–534.
Scott, E. K., Reuter, J. E., & Luo, L. (2003). Small GTPase Cdc42 is required for multiple aspects of dendritic morphogenesis. Journal of Neuroscience, 23(8), 3118–3123.
Segal, M., & Korkotian, E. (2014). Endoplasmic reticulum calcium stores in dendritic spines. Front Neuroanat, 8, 64.
Segal, M., Vlachos, A., & Korkotian, E. (2010). The spine apparatus, synaptopodin, and dendritic spine plasticity. Neuroscientist, 16(2), 125–131.
Segev, I., & Rall, W. (1988). Computational study of an excitable dendritic spine. Journal of Neurophysiology, 60(2), 499–523.
Séguéla, P., Watkins, K. C., & Descarries, L. (1989). Ultrastructural relationships of serotonin axon terminals in the cerebral-cortex of the adult-rat. Journal of Comparative Neurology, 289(1), 129–142.
Séguéla, P., Watkins, K. C., Geffard, M., & Descarries, L. (1990). Noradrenaline axon terminals in adult-rat neocortex: An immunocytochemical analysis in serial thin-sections. Neuroscience, 35(2), 249–264.
Sharp, A. H., McPherson, P. S., Dawson, T. M., Aoki, C., Campbell, K. P., & Snyder, S. H. (1993). Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. Journal of Neuroscience, 13(7), 3051–3063.
Shaw, S. R. (1978). Extracellular-space and blood-eye barrier in an insect retina: Ultrastructural-study. Cell and Tissue Research, 188(1), 35–61.
Shepherd, G. M. (1996). The dendritic spine: A multifunctional integrative unit. Journal of Neurophysiology, 75(6), 2197–2210.
Shepherd, G. M. (2004). The synaptic organization of the brain (5th ed.). New York: Oxford University Press.
Shepherd, G. M., Chen, W. R., Willhite, D., Migliore, M., & Greer, C. A. (2007). The olfactory granule cell: From classical enigma to central role in olfactory processing. Brain Research Reviews, 55(2), 373–382.
Singla, C. L. (1978). Fine structure of the neuromuscular system of Polyorchis penicillatus (Hydromedusae, Cnidaria). Cell and Tissue Research, 193(1), 163–174.
Smith, Y., Bennett, B. D., Bolam, J. P., Parent, A., & Sadikot, A. F. (1994). Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. Journal of Comparative Neurology, 344(1), 1–19.
Smith, C. L., Pivovarova, N., & Reese, T. S. (2015). Coordinated feeding behavior in trichoplax, an animal without synapses. PLoS One, 10(9), e0136098.
Smith, C. L., Varoqueaux, F., Kittelmann, M., Azzam, R. N., Cooper, B., Winters, C. A., et al. (2014). Novel cell types, neurosecretory cells, and body plan of the early-diverging metazoan Trichoplax adhaerens. Current Biology, 24(14), 1565–1572.
Sobkowicz, H. M., Slapnick, S. M., & August, B. K. (2003). Reciprocal synapses between inner hair cell spines and afferent dendrites in the organ of corti of the mouse. Synapse, 50(1), 53–66.
Soghomonian, J. J., Descarries, L., & Watkins, K. C. (1989). Serotonin innervation in adult-rat neostriatum. 2. Ultrastructural features: A autoradiographic and immunocytochemical study. Brain Research, 481(1), 67–86.
Sorra, K. E., & Harris, K. M. (2000). Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus, 10(5), 501–511.
Spacek, J. (1982). ‘Free’ postsynaptic-like densities in normal adult brain: their occurrence, distribution, structure and association with subsurface cisterns. Journal of Neurocytology, 11(5), 693–706.
Spacek, J. (1985). Three-dimensional analysis of dendritic spines. II. Spine apparatus and other cytoplasmic components. Anatomy and Embryology (Berl), 171(2), 235–243.
Spacek, J., & Harris, K. M. (1997). Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. Journal of Neuroscience, 17(1), 190–203.
Spacek, J., & Lieberman, A. R. (1974). Ultrastructure and three-dimensional organization of synaptic glomeruli in rat somatosensory thalamus. Journal of Anatomy, 117(Pt 3), 487–516.
Stefanelli, A., & Caravita, S. (1970). Ultrastructural features of the synaptic complex of the vestibular nuclei of Lampetra planeri (Bloch). Z Zellforsch Mikrosk Anat, 108(2), 282–296.
Stein, I. S., Gray, J. A., & Zito, K. (2015). Non-ionotropic NMDA receptor signaling drives activity-induced dendritic spine shrinkage. Journal of Neuroscience, 35(35), 12303–12308.
Sterling, P., & Demb, J. B. (2004). Retina. In G. M. Shepherd (Ed.), The synaptic organization of the brain (5th ed., pp. 217–269). New York: Oxford University Press.
Sterling, P., & Matthews, G. (2005). Structure and function of ribbon synapses. Trends in Neurosciences, 28(1), 20–29.
Steward, O., & Reeves, T. M. (1988). Protein-synthetic machinery beneath postsynaptic sites on cns neurons: Association between polyribosomes and other organelles at the synaptic site. Journal of Neuroscience, 8(1), 176–184.
Stewart, M. G., Davies, H. A., Sandi, C., Kraev, I. V., Rogachevsky, V. V., Peddie, C. J., et al. (2005). Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: A three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience, 131(1), 43–54.
Stieb, S. M., Muenz, T. S., Wehner, R., & Rossler, W. (2010). Visual experience and age affect synaptic organization in the mushroom bodies of the desert ant Cataglyphis fortis. Developmental Neurobiology, 70(6), 408–423.
Strausfeld, N. J., & Barth, F. G. (1993). 2 Visual systems in one brain: Neuropils serving the secondary eyes of the spider Cupiennius-Salei. Journal of Comparative Neurology, 328(1), 43–62.
Strausfeld, N. J., Strausfeld, C. M., Stowe, S., Rowell, D., & Loesel, R. (2006). The organization and evolutionary implications of neuropils and their neurons in the brain of the onychophoran Euperipatoides rowelli. Arthropod Structure & Development, 35(3), 169–196.
Strausfeld, N. J., Weltzien, P., & Barth, F. G. (1993). 2 visual systems in one brain: Neuropils serving the principal eyes of the spider Cupiennius-Salei. Journal of Comparative Neurology, 328(1), 63–75.
Sullivan, R. K. P., WoldeMussie, E., & Pow, D. V. (2007). Dendritic and synaptic plasticity of neurons in the human age-related macular degeneration retina. Investigative Ophthalmology & Visual Science, 48(6), 2782–2791.
Sutula, T., Cascino, G., Cavazos, J., Parada, I., & Ramirez, L. (1989). Mossy fiber synaptic reorganization in the epileptic human Temporal-Lobe. Annals of Neurology, 26(3), 321–330.
Takahashi, K. (1967). Special somatic spine synapses in ciliary ganglion of Chick. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie, 83(1), 70–75.
Takasaka, T., & Smith, C. A. (1971). The structure and innervation of the pigeon’s basilar papilla. Journal of Ultrastructure Research, 35(1), 20–65.
Tanaka, K., & Smith, C. A. (1978). Structure of the chicken’s inner ear: SEM and TEM study. American Journal of Anatomy, 153(2), 251–271.
Tao-Cheng, J. H., Gallant, P. E., Brightman, M. W., Dosemeci, A., & Reese, T. S. (2007). Structural changes at synapses after delayed perfusion fixation in different regions of the mouse brain. Journal of Comparative Neurology, 501(5), 731–740.
Tardent, P., & Schmid, V. (1972). Ultrastructure of mechanoreceptors of Polyp Coryne-Pintneri (Hydrozoa, Athecata). Experimental Cell Research, 72(1), 265–275.
Tatsuoka, H., & Reese, T. S. (1989). New structural features of synapses in the anteroventral cochlear nucleus prepared by direct freezing and freeze-substitution. Journal of Comparative Neurology, 290(3), 343–357.
Toth, M. L., Melentijevic, I., Shah, L., Bhatia, A., Lu, K., Talwar, A., & Driscoll, M. (2012). Neurite sprouting and synapse deterioration in the aging caenorhabditis Elegans nervous system. Journal of Neuroscience, 32(26), 8778–8790.
Treves, A., Tashiro, A., Witter, M. P., & Moser, E. I. (2008). What is the mammalian dentate gyrus good for? Neuroscience, 154(4), 1155–1172.
Trudeau, L. E., Hnasko, T. S., Wallen-Mackenzie, A., Morales, M., Rayport, S., & Sulzer, D. (2014). The multilingual nature of dopamine neurons. Progress in Brain Research, 211, 141–164.
Trujillo-Cenoz, O. (1965). Some aspects of structural organization of arthropod eye. Cold Spring Harbor Symposia on Quantitative Biology, 30, 371–382.
Trujillo-Cenoz, O., & Melamed, J. (1967). Fine structure of visual system of Lycosa (Araneae - Lycosidae). 2. Primary visual centers. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie, 76(3), 377–388.
Tzingounis, A. V., & Wadiche, J. I. (2007). Glutamate transporters: Confining runaway excitation by shaping synaptic transmission. Nature Reviews Neuroscience, 8(12), 935–947.
Ueno, S., Tsukamoto, M., Hirano, T., Kikuchi, K., Yamada, M. K., Nishiyama, N., et al. (2002). Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-D-aspartate receptor activity in hippocampal CA3 circuits. Journal of Cell Biology, 158(2), 215–220.
Umbriaco, D., Watkins, K. C., Descarries, L., Cozzari, C., & Hartman, B. K. (1994). Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: an electron microscopic study in serial sections. Journal of Comparative Neurology, 348(3), 351–373.
Vaaga, C. E., Borisovska, M., & Westbrook, G. L. (2014). Dual-transmitter neurons: functional implications of co-release and co-transmission. Current Opinion in Neurobiology, 29, 25–32.
Vardi, N., Morigiwa, K., Wang, T. L., Shi, Y. J., & Sterling, P. (1998). Neurochemistry of the mammalian cone ‘synaptic complex’. Vision Research, 38(10), 1359–1369.
Verney, C., Alvarez, C., Geffard, M., & Berger, B. (1990). Ultrastructural double-labelling study of dopamine terminals and GABA-containing neurons in Rat anteromedial cerebral cortex. European Journal of Neuroscience, 2(11), 960–972.
Veruki, M. L., Morkve, S. H., & Hartveit, E. (2006). Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nature Neuroscience, 9(11), 1388–1396.
Vigh, B., Manzano, M. J., Zadori, A., Frank, C. L., Lukats, A., Rohlich, P., & David, C. (2002). Nonvisual photoreceptors of the deep brain, pineal organs and retina. Histology and Histopathology, 17(2), 555–590.
Vlachos, A., Korkotian, E., Schonfeld, E., Copanaki, E., Deller, T., & Segal, M. (2009). Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons. Journal of Neuroscience, 29(4), 1017–1033.
Vogt, K. E., & Nicoll, R. A. (1999). Glutamate and gamma-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proceedings of National Academy of Science USA, 96(3), 1118–1122.
Wainer, B. H., Bolam, J. P., Freund, T. F., Henderson, Z., Totterdell, S., & Smith, A. D. (1984). Cholinergic synapses in the rat brain: A correlated light and electron microscopic immunohistochemical study employing a monoclonal antibody against choline acetyltransferase. Brain Research, 308(1), 69–76.
Wässle, H., Grünert, U., Chun, M. H., & Boycott, B. B. (1995). The rod pathway of the macaque monkey retina: Identification of AII-amacrine cells with antibodies against calretinin. Journal of Comparative Neurology, 361(3), 537–551.
Weber, W., & Grosmann, M. (1977). Ultrastructure of basiepithelial nerve plexus of sea-urchin. Centrostephanus-Longispinus. Cell and Tissue Research, 175(4), 551–562.
Weber, B. H. F., Schrewe, H., Molday, L. L., Gehrig, A., White, K. L., Seeliger, M. W., et al. (2002). Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proceedings of the National Academy of Sciences of the United States of America, 99(9), 6222–6227.
Wellis, D. P., & Kauer, J. S. (1994). Gabaergic and glutamatergic synaptic input to identified granule cells in salamander olfactory-bulb. Journal of Physiology-London, 475(3), 419–430.
Wells, J., Parsons, R. L., Besso, J. A., & Boldosse, W. G. (1972). Fine-structure of nerve cord of Myxicola-Infundibulum (Annelida, Polychaeta). Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie, 131(2), 141–148.
Westfall, J. A. (1996). Ultrastructure of synapses in the first-evolved nervous systems. Journal of Neurocytology, 25(12), 735–746.
Westfall, J. A. (2004). Neural pathways and innervation of cnidocytes in tentacles of sea anemones. Hydrobiologia, 530, 117–121.
White, J. G., Southgate, E., Thomson, J. N., & Brenner, S. (1976). Structure of ventral nerve cord of Caenorhabditis-Elegans. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 275(938), 327–348.
White, J. G., Southgate, E., Thomson, J. N., & Brenner, S. (1986). The structure of the nervous-system of the nematode Caenorhabditis-Elegans. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 314(1165), 1–340.
Whitehead, M. C., & Morest, D. K. (1985). The growth of cochlear fibers and the formation of their synaptic endings in the avian inner ear: A study with the electron microscope. Neuroscience, 14(1), 277–300.
Wiera, G., & Mozrzymas, J. W. (2015). Extracellular proteolysis in structural and functional plasticity of mossy fiber synapses in hippocampus. Frontiers in Cellular Science,. doi:10.3389/fncel.2015.00427.
Wilson, C. J., Murakami, F., Katsumaru, H., & Tsukahara, N. (1987). Dendritic and somatic appendages of identified rubrospinal neurons of the cat. Neuroscience, 22(1), 113–130.
Wolff, G., Harzsch, S., Hansson, B. S., Brown, S., & Strausfeld, N. (2012). Neuronal organization of the hemiellipsoid body of the land hermit crab, Coenobita clypeatus: Correspondence with the mushroom body ground pattern. Journal of Comparative Neurology, 520(13), 2824–2846.
Yamashita, T., Maeda, T., Tokunaga, Y., & Mano, T. (1997). Fine structure of crest synapses in the locus coeruleus of the Japanese macaque (Macaca fuscata), with special reference to noradrenergic neurons. Kaibogaku Zasshi, 72(3), 199–208.
Yamasu, T., & Yoshida, M. (1976). Fine-structure of complex ocelli of a cubomedusan. Tamoya-Bursaria Haeckel. Cell and Tissue Research, 170(3), 325–339.
Yao, W. D., Spealman, R. D., & Zhang, J. (2008). Dopaminergic signaling in dendritic spines. Biochemical Pharmacology, 75(11), 2055–2069.
Yasuyama, K., Meinertzhagen, I. A., & Schurmann, F. W. (2003). Synaptic connections of cholinergic antennal lobe relay neurons innervating the lateral horn neuropile in the brain of Drosophila melanogaster. Journal of Comparative Neurology, 466(3), 299–315.
Yung, K. K., Bolam, J. P., Smith, A. D., Hersch, S. M., Ciliax, B. J., & Levey, A. I. (1995). Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: Light and electron microscopy. Neuroscience, 65(3), 709–730.
Zhang, N. H., & Houser, C. R. (1999). Ultrastructural localization of dynorphin in the dentate gyrus in human temporal lobe epilepsy: A study of reorganized mossy fiber synapses. Journal of Comparative Neurology, 405(4), 472–490.
Zhang, J., Petralia, R. S., Wang, Y.-X., & Diamond, J. S. (2016). High-resolution quantitative immunogold analysis of membrane receptors at retinal ribbon synapses. JoVE, 108(e53547), 1–7.
Zhang, S., Qi, J., Li, X., Wang, H. L., Britt, J. P., Hoffman, A. F., & Morales, M. (2015a). Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nature Neuroscience, 18(3), 386–392.
Zhang, H., Wu, L., Pchitskaya, E., Zakharova, O., Saito, T., Saido, T., & Bezprozvanny, I. (2015b). Neuronal store-operated calcium entry and mushroom spine loss in amyloid precursor protein knock-in mouse model of Alzheimer’s disease. Journal of Neuroscience, 35(39), 13275–13286.
Zhao, S., Studer, D., Chai, X., Graber, W., Brose, N., Nestel, S., et al. (2012). Structural plasticity of hippocampal mossy fiber synapses as revealed by high-pressure freezing. Journal of Comparative Neurology, 520(11), 2340–2351.
Zhao, H. M., Wenthold, R. J., & Petralia, R. S. (1998). Glutamate receptor targeting to synaptic populations on Purkinje cells is developmentally regulated. Journal of Neuroscience, 18(14), 5517–5528.
Zheng, C. Y., Seabold, G. K., Horak, M., & Petralia, R. S. (2011). MAGUKs, synaptic development, and synaptic plasticity. Neuroscientist, 17(5), 493–512.
Acknowledgments
This work was supported by the Intramural Research Programs of NIDCD/NIH and NIA/NIH. The code for the Advanced Imaging Core of NIDCD is ZIC DC000081-03. We thank Megan Wyeth for helpful discussion about hippocampal CA3 mossy terminal synapse function and inhibitory interneurons.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Petralia, R.S., Wang, YX., Mattson, M.P. et al. The Diversity of Spine Synapses in Animals. Neuromol Med 18, 497–539 (2016). https://doi.org/10.1007/s12017-016-8405-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-016-8405-y