Abstract
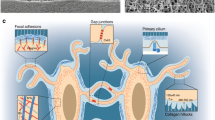
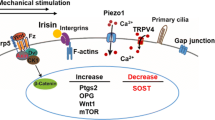
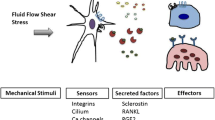
Significant progress has been made in the field of mechanotransduction in bone cells. The knowledge about the role of osteocytes as the professional mechanosensor cells of bone as well as the lacuno-canalicular porosity as the structure that mediates mechanosensing is increasing. New insights might result in a paradigm for understanding the bone formation response to mechanical loading, and the bone resorption response to disuse. Under physiological loading conditions the strain-derived flow of interstitial fluid through the lacuno-canalicular porosity seems to mechanically activate the osteocytes, which subsequently alter the bone remodeling activity of osteoblasts and/or osteoclasts. Fatigue loading results in local microdamage, disruption of normal flow patterns, and osteocyte apoptosis. Apoptotic osteocytes likely attract osteoclasts to resorb the damaged bone. This concept allows explanation of local bone gain and loss, as well as remodeling in response to fatigue damage, as processes supervised by mechanosensitive osteocytes. Uncovering the cellular and mechanical basis of the osteocyte’s response to loading would greatly contribute to our understanding of the cellular basis for bone remodeling, and could contribute to the discovery of new treatment modalities for bone mass disorders, such as osteoporosis.




Similar content being viewed by others
References
Wolff JD. Das Gesetz der Transformation der Knochen. Berlin: A. Hirschwald; 1892.
Cowin SC, Moss-Salentijn L, Moss ML. Candidates for the mechanosensory system in bone. J Biomed Eng. 1991;113:191–7.
Mullender MG, Huiskes R. Proposal for the regulatory mechanism of Wolff’s law. J Orthop Res. 1995;13:503–12.
Mullender MG, Huiskes R. Osteocytes and bone lining cells: which are the best candidates for mechano-sensors in cancellous bone? Bone. 1997;20:527–32.
Klein-Nulend J, Van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9:441–5.
Parfitt AM. The cellular basis of bone turnover and bone loss. Clin Orthop Rel Res. 1977;127:236–47.
Vatsa A, Smit TH, Klein-Nulend J. Extracellular NO signalling from a mechanically stimulated osteocyte. J Biomech. 2007;40:S89–95.
Skerry TM, Bitensky L, Chayen J, Lanyon LE. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. J Bone Miner Res. 1989;4:783–8.
El-Haj AJ, Minter SL, Rawlinson SCF, Suswillo R, Lanyon LE. Cellular responses to mechanical loading in vitro. J Bone Miner Res. 1990;5:923–32.
Dallas SL, Zaman G, Pead MJ, Lanyon LE. Early strain-related changes in cultured embryonic chick tibiotarsi parallel those associated with adaptive modeling in vivo. J Bone Miner Res. 1993;8:251–9.
Lean JM, Jagger CJ, Chambers TJ, Chow JW. Increased insulin-like growth factor I mRNA expression in rat osteocytes in response to mechanical stimulation. Am J Physiol. 1995;268:E318–27.
Forwood MR, Kelly WL, Worth NF. Localization of prostaglandin endoperoxidase H synthase (PGHS)-1 and PGHS-2 in bone following mechanical loading in vivo. Anat Rec. 1998;252:580–6.
Terai K, Takano-Yamamoto T, Ohba Y, Hiura K, Sugimoto M, Sato M, Kawahata H, Inaguma N, Kitamura Y, Nomura S. Role of osteopontin in bone remodeling caused by mechanical stress. J Bone Miner Res. 1999;14:839–49.
McGarry JG, Klein-Nulend J, Prendergast PJ. The effect of cytoskeletal disruption on pulsatile fluid flow-induced nitric oxide and prostaglandin E2 release in osteocytes and osteoblasts. Biochem Biophys Res Commun. 2005;330:341–8.
Tanaka-Kamioka K, Kamioka H, Ris H, Lim SS. Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections. J Bone Miner Res. 1998;13:1555–68.
Aarden EM, Wassenaar AM, Alblas MJ, Nijweide PJ. Immunocytochemical demonstration of extracellular matrix proteins in isolated osteocytes. Histochem Cell Biol. 1996;106:495–501.
Westbroek I, De Rooij KE, Nijweide PJ. Osteocyte-specific monoclonal antibody MAb OB7.3 is directed against Phex protein. J Bone Miner Res. 2002;17:845–53.
Van der Plas A, Nijweide PJ. Isolation and purification of osteocytes. J Bone Miner Res. 1992;7:389–96.
Van der Plas A, Aarden EM, Feyen JHM, de Boer AH, Wiltink A, Alblas MJ, de Ley L, Nijweide PJ. Characteristics and properties of osteocytes in culture. J Bone Miner Res. 1994;9:1697–704.
Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, Simmons HA, Crawford DT, Chidsey-Frink KL, Ke HZ, McNeish JD, Brown TA. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278:1998–2007.
Gluhak-Heinrich J, Yang W, Bonewald L, Robling AG, Turner CH, Harris SE. Mechanically induced DMP1 and MEPE expression in osteocytes: correlation to mechanical strain, osteogenic response and gene expression threshold. J Bone Miner Res. 2005;20(Suppl 1):S73.
Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, Komori T. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res. 2001;16:2017–26.
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–5.
Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, Pavlin D. Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res. 2003;18:807–17.
Harris SE, Gluhak-Heinrich J, Harris MA, Yang W, Bonewald LF, Riha D, Rove PSN, Robling AG, Turner CH, Feng JQ, McKee MD, Nicolella D. DMP1 and MEPE expression are elevated in osteocytes after mechanical loading in vivo: theoretical role in controlling mineral quality in the perilacunar matrix. J Musculoskelet Neuronal Interact. 2007;7:313–5.
Schulze E, Witt M, Kasper M, Löwik CW, Funk RH. Immunohistochemical investigations on the differentiation marker protein E11 in rat calvaria, calvaria cell culture and the osteoblastic cell line ROS 17/2.8. Histochem. Cell Biol. 1999;111:61–9.
Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S, Harris M, Harris SE, Feng JQ, Bonewald LF. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26:4539–52.
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–4.
Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet. 2001;10:537–43.
Van Bezooijen RL, Roelen BA, Visser A, Van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CW. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–14.
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–7.
Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact. 2006;6:354.
Johnson LC. The kinetics of skeletal remodeling in structural organization of the skeleton. Birth Defects. 1966;11:66–142.
Bonucci E. The ultrastructure of the osteocyte. In: Bonucci E, Motta PM, editors. Ultrastructure of skeletal tissues. Dordrecht, The Netherlands: Kluwer Academic; 1990. p. 223–37.
Boyde A. Evidence against “osteocyte osteolysis.”. Metab Bone Dis Rel Res. 1980;2(Suppl):239–55.
Marotti G, Cane V, Palazzini S, Palumbo C. Structure-function relationships in the osteocyte. Ital J Min Electrolyte Metab. 1990;4:93–106.
Ruchon AF, Tenenhouse HS, Marcinkiewicz M, Siegfried G, Aubin JE, DesGroseillers L, Crine P, Boileau G. Developmental expression and tissue distribution of Phex protein: effect of the Hyp mutation and relationship to bone markers. J Bone Miner Res. 2000;15:1440–50.
Oster G. Cell motility and tissue morphogenesis. In: Stein WD, Bronner F, editors. Cell shape: determinants, regulation and regulatory role. San Diego, CA: Academic Press. 1989. p. 33–61.
Pienkowski D, Pollack SR. The origin of stress-generated potentials in fluid-saturated bone. J Orthop Res. 1983;1:30–41.
Rubin CT. Skeletal strain and the functional significance of bone architecture. Calcif Tissue Int. 1984;36:S11–8.
Burr DB, Milgran C, Fyhrie D, Forwood MR, Nyska M, Finestone A, Hoshaw S, Saiag E, Simkin A. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18:405–10.
Vatsa A, Breuls RG, Semeins CM, Salmon PL, Smit TH, Klein-Nulend J. Osteocyte morphology in fibula and calvaria—is there a role for mechanosensing? Bone. (in press).
Vatsa A, Semeins CM, Smit TH, Klein-Nulend J. Paxillin localisation in osteocytes—is it determined by the direction of loading? Biochem Biophys Res Commun. 2008; Jan 7 [Epub ahead of print].
McGarry JG, Klein-Nulend J, Mullender MG, Prendergast PJ. A comparison of strain and fluid shear stress in stimulating bone cell responses—a computational and experimental study. FASEB J. 2005;19:482–4.
Cowin SC, Weinbaum S, Zeng Y. A case for bone canaliculi as the anatomical site of strain generated potentials. J Biomech. 1995;28:1281–97.
Cowin SC. Bone poroelasticity. J Biomech. 1999;32:217–38.
Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–60.
Knothe-Tate ML, Steck R, Forwood MR, Niederer P. In vivo demonstration of load-induced fluid flow in the rat tibia and its potential implications for processes associated with functional adaptation. J Exp Biol. 2000;203:2737–45.
Burger EH, Klein-Nulend J. Mechanotransduction in bone: role of the lacuno-canalicular network. FASEB J. 1999;13:S101–12.
You J, Yellowley CE, Donahue HJ, Zhang Y, Chen Q, Jacobs CR. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loadinginduced oscillating fluid flow. J Biomech Engin. 2000;122:387–93.
Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269:80–2.
Knothe Tate ML, Knothe U, Niederer P. Experimental elucidation of mechanical load-induced fluid flow and its potential role in bone metabolism and functional adaptation. Am J Med Sci. 1998;316:189–95.
Pollack SR, Petrov N, Salzstein R, Brankov G, Blagoeva R. An anatomical model for streaming potentials in osteons. J Biomech. 1984;17:627–36.
Salzstein RA, Pollack SR. Electromechanical potentials in cortical bone. II. Experimental analysis. J Biomech. 1987;20:271–80.
Hung CT, Pollack SR, Reilly TM, Brighton CT. Realtime calcium response of cultured bone cells to fluid flow. Clin Orthop Rel Res. 1995;313:256–69.
Hung CT, Allen FD, Pollack SR, Brighton CT. Intracellular calcium stores and extracellular calcium are required in the real-time calcium response of bone cells experiencing fluid flow. J Biomech. 1996;29:1411–7.
Hung CT, Allen FD, Pollack SR, Brighton CT. What is the role of the convective current density in the real-time calcium response of cultured bone cells to fluid flow? J Biomech. 1996;29:1403–9.
Wang N, Ingber DE. Control of cytoskeletal mechanisms by extracellular matrix, cell shape and mechanical tension. Biophys J. 1994;66:2181–9.
Sachs F. Ion channels as mechanical transducers. In: Stein WD, Bronner F, editors. Cell shape: determinants, regulation and regulatory role. San Diego, CA: Academic Press. 1989. p. 63–94.
Ingber DE. Intergrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3:841–8.
Bakker AD, Soejima K, Klein-Nulend J, Burger EH. The production of nitric oxide and prostaglandin E2 by primary bone cells is shear stress dependent. J Biomech. 2001;34:671–7.
Kamiya A, Ando J. Response of vascular endothelial cells to fluid shear stress: mechanism. In: Hayashi K, Kamiyn A, Ono K, editors. Biomechanics: functional adaptation and remodeling. Tokyo: Springer; 1996. p. 29–56.
Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–9.
Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts-correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217:640–8.
Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheid-Lammers T, Alblas MJ, Burger EH. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes—a cytoskeleton-dependent process. Biochem Biophys Res Commun. 1996;225:62–8.
Westbroek I, Ajubi NE, Alblas MJ, Semeins CM, Klein-Nulend J, Burger EH, Nijweide PJ. Differential stimulation of prostaglandin G/H synthase-2 in osteocytes and other osteogenic cells by pulsating fluid flow. Biochem Biophys Res Commun. 2000;268:414–9.
Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993;260:1124–7.
Watson PA. Function follows form: generation of intracellular signals by cell deformation. FASEB J. 1991;5:2013–9.
Ajubi NE, Klein-Nulend J, Alblas MJ, Burger EH, Nijweide PJ. Signal transduction pathways involved in fluid flow-induced prostaglandin E2 production by cultured osteocytes. Am J Physiol. 1999;276:E171–8.
Pavalko FM, Chen NX, Turner CH, Burr DB, Atkinson S, Hsieh YF, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeletonintegrin interactions. Am J Physiol. 1998;275:C1591–601.
Aarden EM, Nijweide PJ, Van der Plas A, Alblas MJ, Mackie EJ, Horton MA, Helfrich MH. Adhesive properties of isolated chick osteocytes in vitro. Bone. 1996;18:305–13.
Hughes DE, Salter DM, Simpson R. CD44 expression in human bone: a novel marker of osteocytic differentiation. J Bone Miner Res. 1994;9:39–44.
Nakamura H, Ozawa H. Immunolocalization of CD44 and the ERM family in bone cells of mouse tibiae. J Bone Miner Res. 1996;11:1715–22.
Bacabac RG, Smit TH, Mullender MG, Dijcks SJ, Van Loon JJWA, Klein-Nulend J. Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem Biophys Res Commun. 2004;315:823–9.
Bacabac RG, Smit TH, Van Loon JJWA, Zandieh Doulabi B, Helder MN, Klein-Nulend J. Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm? FASEB J. 2006;20:858–64.
Mullender MG, Dijcks SJ, Bacabac RG, Semeins CM, Van Loon JJWA, Klein-Nulend J. Release of nitric oxide, but not prostaglandin E2, by bone cells depends on fluid flow frequency. J Orthop Res. 2006;24:1170–7.
Bacabac RG, Smit TH, Mullender MG, Van Loon JJWA, Klein-Nulend J. Initial stress-kick is required for fluid shear stress-induced rate dependent activation of bone cells. Ann Biomed Eng. 2005;33:104–10.
Bacabac RG, Mizuno D, Schmidt CF, MacKintosh FC, Smit TH, Van Loon JJWA, Klein-Nulend J. Microrheology and force traction of mechanosensitive bone cells. J Biomech. 2006;39(Suppl 1):S231–2.
Vatsa A, Mizuno D, Smit TH, Schmidt CF, MacKintosh FC, Klein-Nulend J. Bio imaging of intracellular NO production in single bone cells after mechanical stimulation. J Bone Miner Res. 2006;21:1722–8.
Bacabac RG, Mizuno D, Schmidt CF, MacKintosh FC, Van Loon JJWA, Klein-Nulend J, Smit TH. Bone cell morphology, elasticity, and mechanosensing. J Biomech. 2008; Apr 8 [Epub ahead of print].
Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald LF, Quarles LD. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem. 2006;281:30884–95.
Malone AMD, Anderson CT, Temiyasathit S, Tang J, Tummala P, Sterns T, Jacobs CR. Primary Cilia: Mechanosensory Organelles in bone cells. J Bone Miner Res. 2006;21(Suppl 1):S39.
Doty SB. Morphological evidence of gap junctions between bone cells. Calcif Tissue Int. 1981;33:509–12.
Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem 1996;65:475–502.
Bennett MV, Goodenough DA. Gap junctions, electrotonic coupling, and intercellular communication. Neurosci Res Program Bull. 1978;16:1–486.
Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931–44.
Saunders MM, You J, Trosko JE, Yamasaki H, Li Z, Donahue HJ, Jacobs CR. Gap junctions and fluid flow response in MC3T3–E1 cells. Am J Physiol Cell Physiol. 2001;281:C1917–25.
Cheng B, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J Bone Miner Res. 2001;16:249–59.
Alford AI, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism small star, filled. Bone. 2003;33:64–70.
Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 1997;12:2014–23.
Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–94.
Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces atp release from MLO-Y4 osteocytes. J Cell Physiol. Published online 2007 Feb 14 [Epub ahead of print].
Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–57.
Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–6.
Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci USA. 2004;101:16689–94.
Wang Y, McNamara LM, Schaffler MB, Weinbaum S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci USA. 2007;104:15941–846.
Veno P, Nicolella DP, Sivakumar P, Kalajzic I, Rowe D, Harris SE, Bonewald LF, Dallas SL. Live imaging of osteocytes within their lacunae reveals cell body and dendrite motions. J Bone Min Res. 2006;21(Suppl 1):S38.
Chen NX, Ryder KD, Pavalko FM, Turner CH, Burr DB, Qiu J, Duncan RL. Ca(2+) regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am J Physiol. 2000;278:C989–97.
Klein-Nulend J, Burger EH, Semeins CM, Raisz LG, Pilbeam CC. Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J Bone Miner Res. 1997;12:45–51.
Joldersma M, Burger EH, Semeins CM, Klein-Nulend J. Mechanical stress induces COX-2 mRNA expression in bone cells from elderly women. J Biomech. 2000;33:53–61.
Forwood MR. Inducible cyclooxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res. 1996;11:1688–93.
Pitsillides AA, Rawlinson SCF, Suswillo RFL, Bourrin S, Zaman G, Lanyon LE. Mechanical strain-induced NO production by bone cells: a possible role in adaptive bone (re)modeling? FASEB J. 1995;9:1614–22.
Sterck JGH, Klein-Nulend J, Lips P, Burger EH. Response of normal and osteoporotic human bone cells to mechanical stress in vitro. Am J Physiol. 1998;274:E1113–20.
Turner CH, Takano Y, Owan I, Murrell GA. Nitric oxide inhibitor L-NAME suppresses mechanically induced bone formation in rats. Am J Physiol. 1996;270:E639–43.
Fox SW, Chambers TJ, Chow JWM. Nitric oxide is an early mediator of the increase in bone formation by mechanical stimulation. Am J Physiol. 1996;270:E955–60.
Koprowski H, Maeda H. The role of nitric oxide in physiology and pathophysiology. Berlin, Germany: Springer-Verlag; 1995.
Helfrich MH, Evans DE, Grabowski PS, Pollock JS, Ohshima H, Ralston SH. Expression of nitric oxide synthase isoforms in bone and bone cell cultures. J Bone Miner Res. 1997;12:1108–15.
Zaman G, Pitsillides AA, Rawlinson SC, Suswillo RF, Mosley JR, Cheng MZ, Platts LA, Hukkanen M, Polak JM, Lanyon LE. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res. 1999;14:1123–31.
Klein-Nulend J, Helfrich MH, Sterck JGH, MacPherson H, Joldersma M, Ralston SH, Semeins CM, Burger EH. Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem Biophys Res Commun. 1998;250:108–14.
Caballero-Alias AM, Loveridge N, Lyon A, Das-Gupta V, Pitsillides A, Reeve J. NOS isoforms in adult human osteocytes: multiple pathways of NO regulation? Calcif Tissue Int. 2004;75:78–84.
Busse R, Fleming I. Pulsatile stretch and shear stress: physical stimuli determining the production of endothelium derived relaxing factors. J Vascul Res. 1998;35:73–84.
Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial nitric oxide synthase mRNA expression by shear stress. Am J Physiol. 1995;269:C1371–8.
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810.
Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–30.
Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–5.
Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–3.
Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309.
Johnson ML, Harnish K, Nusse R, Van Hul W. LRP5 and Wnt signaling: a union made for bone. J Bone Miner Res. 2004;19:1749–57.
Lories RJ, Peeters J, Bakker AD, Tylzanowski P, Derese I, Schrooten J, Thomas JT, Luyten FP. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007;56:3881–3.
Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–8.
Santos A, Bakker AD, Zandieh-Doulabi B, Semeins CM, Klein-Nulend J. Pulsating fluid flow modulates gene expression of proteins involved in Wnt signaling pathways in osteocytes. Transactions of the 54th Annual Meeting, Orthopaedic Research Society, vol 33, abstract, 2008.
Vezeridis PS, Semeins CM, Chen Q, Klein-Nulend J. Osteocytes subjected to pulsating fluid flow regulate osteoblast proliferation and differentiation. Biochem Biophys Res Commun. 2005;348:1082–88.
Tan SD, de Vries TJ, Kuijpers-Jagtman AM, Semeins CM, Everts V, Klein-Nulend J. Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone. 2007;41:745–51.
Mishra S, Knothe-Tate ML. Effect of lacunocanalicular architecture on hydraulic conductance in bone tissue: implications for bone health and evolution. Anat Rec A Discov Mol Cell Evol Biol. 2003;273:752–62.
Tami AE, Nasser P, Verborgt O, Schaffler MB, Knothe Tate ML. The role of interstitial fluid flow in the remodeling response to fatigue loading. J Bone Miner Res. 2002;17:2030–7.
Burger EH, Klein-Nulend J, Smit TH. Strain-derived canalicular fluid flow regulates osteoclast activity in a remodelling osteon—a proposal. J Biomech. 2003;36:1453–9.
Smit TH, Burger EH, Huyghe JM. A case for strain-induced fluid flow as a regulator of BMU-coupling and osteonal alignment. J Bone Miner Res. 2002;17:2021–2029.
Bakker AD, Klein-Nulend J, Burger EH. Shear stress inhibits while disuse promotes osteocyte apoptosis. Biochem Biophys Res Commun. 2004;320:1163–8.
Tan SD, Kuijpers-Jagtman AM, Semeins CM, Bronckers ALJJ, Maltha JC, Von den Hoff JW, Everts V, Klein-Nulend J. Fluid shear stress inhibits TNFalpha-induced osteocyte apoptosis. J Dent Res. 2006;85:905–9.
Basso N, Heersche JNM. Effects of hind limb unloading and reloading on nitric oxide synthase expression and apoptosis of osteocytes and chondrocytes. Bone. 2006;39:807–14.
Lanyon LE. Functional strain as a determinant for bone remodeling. Calcif Tissue Int. 1984;36(Suppl 1):S56–61.
McCreadie BR, Hollister SJ, Schaffler MB, Goldstein SA. Osteocyte lacuna size and shape in women with and without osteoporotic fracture. J Biomech. 2004;37:563–72.
Bakker AD, Klein-Nulend J, Tanck E, Heyligers IC, Albers GH, Lips P, Burger EH. Different responsiveness to mechanical stress of bone cells from osteoporotic versus osteoarthritic donors. Osteoporos Int. 2006;17:827–33.
Bakker AD, Klein-Nulend J, Tanck E, Albers GH, Lips P, Burger EH. Additive effects of estrogen and mechanical stress on nitric oxide and prostaglandin E2 production by bone cells from osteoporotic donors. Osteoporos Int. 2005;16:983–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klein-Nulend, J., Bakker, A.D. Osteocytes: Mechanosensors of Bone and Orchestrators of Mechanical Adaptation. Clinic Rev Bone Miner Metab 5, 195–209 (2007). https://doi.org/10.1007/s12018-008-9014-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-008-9014-6