Abstract
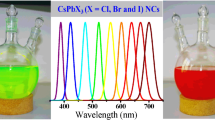
All-inorganic caesium lead-halide perovskite \(\hbox {CsPbBr}_{3}\) and \(\hbox {CsPb}_{2}\hbox {Br}_{5}\) powders have emerged as attractive optoelectronic materials owing to their stabilities and highly efficient photoluminescence (PL). Herein we report a facile chemical route to prepare highly luminescent monoclinic \(\hbox {CsPbBr}_{3}\) and tetragonal \(\hbox {CsPb}_{2}\hbox {Br}_{5}\) powders at room temperature. The \(\hbox {CsPbBr}_{3}\) powders exhibit regular crystal shape and demonstrate polyhedral geometry with an average particle size of 10 \(\upmu \)m. The \(\hbox {CsPb}_{2}\hbox {Br}_{5}\) powders show platelet morphologies and the lateral sizes of the particles are from 5 up to 200 \(\upmu \)m. Both \(\hbox {CsPbBr}_{3}\) and \(\hbox {CsPb}_{2}\hbox {Br}_{5}\) powders present a narrow emission line-width and PL emission of 528 and 527 nm, respectively. A direct band gap of 2.35 eV and an indirect band gap of 3.01 eV are calculated for \(\hbox {CsPbBr}_{3}\) and \(\hbox {CsPb}_{2}\hbox {Br}_{5}\) powders, respectively. In addition, the monoclinic \(\hbox {CsPbBr}_{3}\) can be transformed to tetragonal \(\hbox {CsPb}_{2}\hbox {Br}_{5}\) in the presence of water. The large-scale synthesis of \(\hbox {CsPbBr}_{3}\) and \(\hbox {CsPb}_{2}\hbox {Br}_{5}\) will be advantageous in future applications of optoelectronic devices.










Similar content being viewed by others
References
Dong Q, Fang Y, Shao Y, Mulligan P, Qiu J, Cao L and Huang J 2015 Science 347 967
Noh J H, Im S H, Heo J H, Mandal T N and Seok S I 2013 Nano Lett. 13 1764
Liu M, Johnston M B and Snaith H J 2013 Nature 501 395
Yang W S, Noh J H, Jeon N J, Kim Y C, Ryu S, Seo J et al 2015 Science 348 1234
Tan Z K, Moghaddam R S, Lai M L, Docampo P, Higler R, Deschler F et al 2014 Nat. Nanotechnol. 9 687
Kim Y H, Cho H, Heo J H, Kim T S, Myoung N, Lee C L et al 2015 Adv. Mater. 27 1248
Zhang Q, Ha S T, Liu X, Sum T C and Xiong Q 2014 Nano Lett. 14 5995
Sutherland B R, Hoogland S, Adachi M M, Wong C T and Sargent E H 2014 ACS Nano 8 10947
Hu X, Zhang X, Liang L, Bao J, Li S, Yang W et al 2014 Adv. Funct. Mater. 24 7373
Dou L, Yang Y M, You J, Hong Z, Chang W H, Li G et al 2014 Nat. Commun. 5 5404
Zhu Z, Hadjiev V G, Rong Y, Guo R, Cao B, Tang Z et al 2016 Chem. Mater. 28 7385
Gu Z, Wang K, Sun W, Li J, Liu S, Song Q et al 2016 Adv. Opt. Mater. 4 472
Song J, Xu L, Li J, Xue J, Dong Y, Li X et al 2016 Adv. Opt. Mater. 28 4861
Li X, Wu Y, Zhang S, Cai B, Gu Y, Song J et al 2016 Adv. Funct. Mater. 26 2435
Swarnkar A, Chulliyil R, Ravi V K, Irfanullah M, Chowdhury A and Nag A 2015 J Angew. Chem. Int. Ed. 54 15424
Kulbak M, Cahen D and Hodes G 2015 J. Phys. Chem. Lett. 6 2452
Pan J, Sarmah S P, Murali B, Dursun I, Peng W, Parida M R et al 2015 J. Phys. Chem. Lett. 6 5027
Song J, Li J, Li X, Xu L, Dong Y and Zeng H 2015 Adv. Mater. 27 7162
Zhang X, Xu B, Zhang J, Gao Y, Zheng Y, Wang K et al 2016 Adv. Funct. Mater. 26 4595
Tang X, Hu Z, Yuan W, Hu W, Shao H, Han D et al 2017 Adv. Opt. Mater. 5 1600788
Akkerman Q A, Motti S G, Kandada A R S, Mosconi E, D’Innocenzo V, Bertoni G et al 2016 J. Am. Chem. Soc. 138 1010
Bekenstein Y, Koscher B A, Eaton S W, Yang P and Alivisatos A P 2015 J. Am. Chem. Soc. 137 16008
Rodová M, Brožek J, Knížek K and Nitsch K 2003 J. Therm. Anal. Calorim. 71 667
Møller C K 1958 Nature 182 1436
Protesescu L, Yakunin S, Bodnarchuk M I, Krieg F, Caputo R, Hendon C H et al 2015 Nano Lett. 15 3692
Shamsi J, Dang Z, Bianchini P, Canale C, Stasio F D, Brescia R et al 2016 J. Am. Chem. Soc. 138 7240
Ma R, Liu Z, Takada K, Iyi N, Bando Y and Sasaki T 2007 J. Am. Chem. Soc. 129 5257
Niu G D, Guo X D and Wang L D 2015 J. Mater. Chem. A 3 8970
Tang X, Hu Z, Chen W, Xing X, Zang Z, Hu W et al 2016 Nano Energy 28 462
Li G, Wang H, Zhu Z, Chang Y, Zhang T, Song Z et al 2016 Chem. Commun. 52 11296
Ruan L, Shen W, Wang A, Xiang A and Deng Z 2017 J. Phys. Chem. Lett. 8 3853
Acknowledgements
This work was supported by the China Postdoctoral Science Foundation under Grant Number 2015M582584, the Postdoctoral Research Project of Shaanxi Province under Grant Number 2016BSHEDZZ06, the Special Fund for Basic Scientific Research of Central Colleges, Chang’an University, under Grant Number 310831171011 and the Special Fund for Basic Research Support Programs of Chang’an University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, X., Zhang, J. & Bai, G. Facile synthesis and characterization of \(\hbox {CsPbBr}_{3}\) and \(\hbox {CsPb}_{2}\hbox {Br}_{5}\) powders. Bull Mater Sci 41, 38 (2018). https://doi.org/10.1007/s12034-018-1566-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-018-1566-6