Abstract
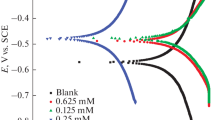
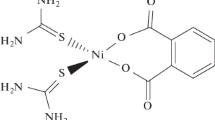
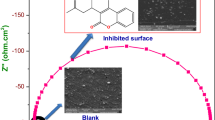
Synergistic inhibition of corrosion of carbon steel in low chloride aqueous medium using tungstate as a synergist in combination with N,N-bis(phosphonomethyl) glycine (BPMG) and zinc ions is presented. The synergistic action of tungstate has been established through the present studies. The new ternary inhibitor formulation is effective in neutral and slightly acidic as well as slightly alkaline media. Potentiodynamic polarisation studies inferred that the formulation functions as a mixed inhibitor. Impedance studies of the metal/solution interface revealed that the surface film is highly protective. Characterisation by X-ray photoelectron spectroscopy (XPS) of the surface film formed in presence of the inhibitor revealed the presence of iron, phosphorus, nitrogen, oxygen, carbon, zinc and tungsten in the surface film. The chemical shifts in the binding energies of these elements inferred that the surface film is composed of iron oxides/hydroxides, zinc hydroxide, heteropolynuclear complex [Fe(III), Zn(II)-BPMG] and WO3. Reflection absorption FTIR spectroscopic studies also supported the presence of these compounds in the surface film. Morphological features of the metal surface studied in the absence and presence of the inhibitor by scanning electron microscopy (SEM) are also presented. Based on all these results, a plausible mechanism of corrosion inhibition is proposed.
Similar content being viewed by others
References
Gunasekaran G, Palaniswamy N, Appa Rao B V and Muralidharan V S 1996 Proc. Indian Acad. Sci. (Chem. Sci.) 108 399
Gonzalez Y, Lafont M C, Pebere N and Moran F 1996 J. Appl. Electrochem. 26 1259
Shaban A, Kalman E and Biczo I 1993 Corros. Sci. 35 1463
Felhosi I, Keresztes Zs, Karman F H, Mohai M, Bertoti I and Kalman E 1999 J. Electrochem. Soc. 146 961
Pech-Canul M A and Chi-Canul L P 1999 Corrosion 55 948
Telegdi J, Shaglouf M M, Shaban A, Karman F H, Betroti I, Mohai M and Kalman E 2001 Electrochim. Acta 46 3791
Ochoa N, Baril G, Moran F and Pebere N 2002 J. Appl. Electrochem. 32 497
Pech-Canul M A and Bartolo-Perez P 2004 Surf. Coat. Technol. 184 133
Appa Rao B V, Srinivasa Rao S and Venkateswara Rao M 2008 Corros. Eng. Sci. Technol. 43 46
Westerback S, Rajan K S and Martell A E 1965 J. Am. Chem. Soc. 87 2567
Sawada K, Duan W, Ono M and Satoh K 2000 J. Chem. Soc., Dalton Trans. 919
Lumsden J B and Szklarska-Smiralowska Z 1978 Corrosion 34 167
Mu G, Li X, Qu Q and Zhou J 2006 Corros. Sci. 48 445
Qu Q, Li L, Bai W, Jiang S and Ding Z 2009 Corros. Sci. 51 2423
ASTM Standard G31-72 1999 (2004) Standard practice for laboratory immersion corrosion testing of metals, ASTM International, West Conshohocken, PA 2006 DOI: 10.1520/G0031-72R04.
Elachouri E, Hajji M S, Salem M, Kertit S, Aride J, Coudert R and Essassi E 1996 Corrosion 52 103
Aravio-Torre J and Arevalo A 1951 Inst. Espam. Oceanogr. 46 27
Morad M S 2000 Corros. Sci. 42 1307
Gunasekaran G and Chauhan L R 2004 Electrochim. Acta 49 4387
Alagta A, Felhosi I, Telegdi J, Bertoti I and Kalman E 2007 Corros. Sci. 49 2754
Wang C T, Chen S H, Ma H Y and Wang N X 2002 J. Serb. Chem. Soc. 67 685
Kalman E, Karman F H, Cserny I, Kover L, Telegdi J and Varga D 1994 Electrochim. Acta 39 1179
McIntyre N S and Zetaruk D G 1977 Anal. Chem. 49 1521
Maroie S, Savy M and Verbist J J 1979 Inorg. Chem. 18 2560
Asami K, Hashimoto K and Shimodaira S 1976 Corros. Sci. 16 35
Moulder J F, Stickle W F, Sobol P E and Bamben K D 1995 Handbook of X-ray photoelectron spectroscopy: a reference book of standard spectra for identification and interpretation of XPS data, USA, Physical Electronics
Nakayama N 2000 Corros. Sci. 42 1897
Koudelka M, Sanchez J and Augustynski J 1982 J. Electrochem. Soc. 129 1186
El Azhar M, Traisnel M, Mernari B, Gengembre L, Bentiss F and Lagrenee M 2002 Appl. Surf. Sci. 185 197
Meneguzzi A, Ferreira C A, Pham M C, Delamar M and Lacaze P C 1999 Electrochim. Acta 44 2149
Aramaki K and Shimura T 2003 Corros. Sci. 45 2639
Fang J L, Li Y, Ye X R, Wang Z W and Liu Q 1993 Corrosion 49 266
Aramaki K 2004 Corros. Sci. 46 1565
Sastri V S 1998 Corrosion inhibitors-principles and applications (England: John Wiley & Sons)
Sastri V S and Packwood R H 1987 Werkstoffe and Korrosion 38 77
Amar H, Braisaz T, Villemin D and Moreau B 2008 Mater. Chem. Phys. 110 1
To X H, Pebere N, Pelaprat N, Boutevin B and Hervaud Y 1997 Corros. Sci. 39 1925
Carter R O, Gierczak C A and Dickie R A 1986 Appl. Spectrosc. 40 649
Sekine I and Hirakawa Y 1986 Corrosion 42 272
Gunasekaran G, Palaniswamy N, Appa Rao B V and Muralidharan V S 1997 Electrochim. Acta 42 1427
Koltypin Yu, Nikitenko S I and Gedanken A 2002 J. Mater. Chem. 12 1107
Shi Yu and Gan Moog Chow 2004 J. Mater. Chem. 14 2781
Bellamy L J 1968 Advances in infrared group frequencies (Great Britain, The Chaucer Press Limited)
Pak J-J, Bahgat M and Paek M-K 2009 J. Alloy Compd. 477 357
Deluchat V, Bollinger J-C, Serpaud B and Caullet C 1997 Talanta 44 897
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Appa Rao, B.V., Venkateswara Rao, M., Srinivasa Rao, S. et al. Tungstate as a synergist to phosphonate-based formulation for corrosion control of carbon steel in nearly neutral aqueous environment. J Chem Sci 122, 639–649 (2010). https://doi.org/10.1007/s12039-010-0099-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-010-0099-3