Abstract
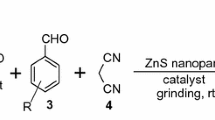
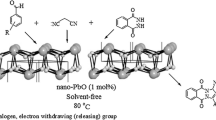
PbO nanoparticles have been employed as an efficient catalyst for the solvent-free synthesis of tetrahydrobenzo pyrans (yields 81–91%) and benzylidene malonitriles (yields 90–96%) at room temperature using green chemistry approach. PbO nanoparticles were found to be highly efficient, eco-friendly and recyclable heterogeneous catalyst. PbO nanoparticles were prepared by hydrothermal method and characterized by IR, XRD, BET Surface area, SEM, EDAX and TEM with SAED techniques.

One-pot synthesis of tetrahydro benzo pyrans and benzylidene malonitriles using PbO nanoparticle under solvent-free condition at room temperature has been presented. PbO nanoparticles were found to be highly efficient, ecofriendly and recyclable catalyst.








Similar content being viewed by others
References
a) Montandon J B, Zijlstra F J and Wilson J H P 1989 Int. J. Tissue React. 11 107; b) Brooks G T 1998 J. Pestic. Sci. 22 41
a) Hafez E A A, Elnagdi M H, Elagamey A G A and EL-Taweel F M A 1987 Heterocycles 26 903; b) Abdel Galil F M, Riad B Y, Sherif S M and Elnagdi M H 1982 Chem. Let. 11(8) 1123
Osherov N, Gazit A, Gilon C and Levitzki A 1993 J. Bio. Chem. 268 11134
Kamath S and Buolamwini J K 2003 J. Med. Chem. 46(22) 4657
Li R, Pourpak A and Morris S W 2010 J. Med. Chem. 52(16) 4981
Lee C T, Adachi Y and Carbone D P 2005 Gene. Ther. Mol. Biol. 9 77
Pore D M, Undale K A and Dongare B B 2009 Catal. Lett. 132(1–2) 104
Katkar S S, Lande M K, Arbad B R and Gaikwad S T 2011 Chin. J. Chem. 29(1) 199
Islami D M and Mosaddegh E 2009 Phosphorus Sulfur Silicon Related Elem. 184(12) 3134
Balalaie S, Bararjanian M, Amani A M and Movassagh B 2006 Synlett 2 263
Oskooie H A, Heravi M M, Karimi N and Zadeh M E 2011 Synth. Commun. 41(3) 436
Jin T S, Wang A Q, Wang X, Zhang J S and Li T S 2004 Synlett 5 871
a) Ziarani G M, Abbasi A, Badiei A and Aslani Z 2011 E-J. Chem. 8(1) 293; b) Bahareh S, Alireza H and Somayeh B 2011 J. Chem. Res. 35(11) 666
a) Gurumurthi S, Sundari V and Vallippan R 2009 E-J. Chem. 6(S1) S 466; b) Mobinikhaledi A and Fard M A 2010 Acta. Chim. Slov. 57 931
a) Wang L M, Shao J H, Tian H, Wang Y H and Liu B 2006 J. Fluorine Chem. 127 97; b) Mei H and Chun C 2010 J. Chem. Res. 34(10) 568
Zhao L, Li Y, Chen L and Zhou B 2010 J. Org. Chem. 30(1) 124
Nemouchi S, Boulcina R, Carboni B and Debache A 2012 Comptes Rendus Chime. 15(5) 394
a) Saini A, Kumar S and Sandhu J S 2006 Synlett 12 1928; b) Sun W B, Zhang P, Fan J, Chen S H and Zhang Z H 2010 Synth. Commun. 10(4) 587
Balaskar R, Gavade S, Mane M, Pabrekar P, Shingare M and Mane D 2011 Lett. Org. Chem. 8(4) 282
Feng C, Wang Q, Lu C, Yang G and Chen Z 2012 Combinatorial Chem. High Throughput Screening 15(1) 100
Ranu B C, Banerjee S and Roy S 2008 Indian J. Chem. 47B 1108
Yu L Q, Liu F and You Q D 2009 Org. Prep. Proc. Int. 41 77
a) Balalaie S, Bararjanian M, Ahmadi M S, Hekmat S and Salehi P 2007 Synth. Commun. 37(7) 1097; b) Abdolmohammadi S and Balalaie S 2007 Tetrahedron Lett. 48 3299
Peng Y and Song G 2003 Indian J. Chem. 42B 924
Shi D Q, Chen J, Zhuang Q Y, Wang X S and Hu H W 2003 Chin. Chem. Lett. 14(12) 1242
Ye W, Jiang H and Yang X C 2011 J. Chem. Sci. 123(3) 331
Parida K M, Rath D and Mol J 2009 J. Mol. Catal. A: Chem. 310 93
Mogilaiah K, Babu S H, Vidya K and Kumar K S 2010 Indian J. Chem. 49B 390
Abaee M S, Mojtahedi M M, Zahedi M M and Khanalizadeh G 2006 Arkivoc xv 48
a) Cardillo G, Fabbrani S, Gentilucci L, Gianotti M and Tolomelli A 2003 Synth. Commun. 33 1587; b) Wang Y, Shang Z, Wu T, Fan J and Chen X J 2006 J. Mol. Catal. A: Chem. 253 212
a) Wang S, Ren Z, Cao W and Tong W 2001 Synth. Commun. 31 673; b) Jin T S, Wang X, Liu L B and Li T S 2006 J. Chem. Res. 6 346
Heravi M M, Bakhtiari K, Taheri and Oskooie H A 2007 J. Chin. Chem. Soc. 54 1557
Su F, Antonietti M and Wang X 2012 Catal. Sci. Technol. 2 1005
Mondal J, Modak A and Bhaumik A 2011 J. Mol. Catal. A: Chem. 335(1–2) 236
Reddy B M, Patil M K, Rao K N and Reddy G K 2006 J. Mol. Catal. A: Chem. 258(1–2) 302
Isobe K, Hoshi T, Suzuki T and Hagiwara H 2005 Mol. Divers. 9(4) 317
Yuan S, Li Z and Xu L 2012 Res. Chem. Intermed. 38(2) 393
Kumar B V, Naik H S, Girija D and Kumar B V 2011 J. Chem. Sci. 123(5) 615
Pasha S K, Satyanarayana V S V, Sivakumar A, Chidambaram K and Kennedy L J 2011 Chin. Chem. Lett. 22(8) 891
Bytyn W and Baerns M 1986 Appl. Catal. 28 199
Dash P K and Balto Y 2011 Res. J. Nanosci. Nanotechnol. 1 25
Borhade A V, Tope D R and Patil D R 2012 Res. Chem. Intermed. doi:10.1007/s11164-012-0693-8
Borhade A V, Tope D R and Patil D R 2012 J. Chem. Pharm. Res. 4(5) 2501
Acknowledgements
Authors thank the University Grants Commission (UGC), New Delhi, for financial support, University of Pune, Pune and Sophisticated Analytical Instrument Facility (SAIF) Panjab University, Chandigarh for providing spectral analysis facilities. Authors are also thankful to Prof. A G Gadhave for his helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary material
Supplementary material given as figures S1–S5 can be seen online www.ias.ac.in/chemsci website.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
BORHADE, A.V., UPHADE, B.K. & TOPE, D.R. PbO as an efficient and reusable catalyst for one-pot synthesis of tetrahydro benzo pyrans and benzylidene malonitriles. J Chem Sci 125, 583–589 (2013). https://doi.org/10.1007/s12039-013-0396-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0396-8