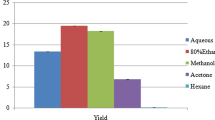
Abstract
The present investigation is focused on the study of chemical composition of a bioactive compound derived from a rumen isolate Paracoccus pantotrophus FMR19 using GC–MS and to find out the antibacterial activity of the extracted crude bioactive compounds against multidrug resistant organisms (MDROs) and other clinical pathogens. GC–MS analysis revealed that P. pantotrophus FMR19 produced eight major compounds that have been reported to exhibit antimicrobial property. The main components identified from hexane fraction are long chain alkanes, fatty alcohols, fatty acid methyl ester and aromatic hydrocarbons. These molecules are not only active against clinical pathogens such as Salmonella sp. and Proteus sp. and also effective against MDROs such as Metallo β lactamase and Pan drug resistant bacterial strains and Methicillin resistant Staphylococcus aureus.



Similar content being viewed by others
References
Atta HM, Dabour SM, Desoukey SG (2009) Sparsomycin antibiotic production by Streptomyces sp. AZ-NIOFD1: taxonomy, fermentation, purification and biological activities. Am Eurasian J Agric Environ Sci 5:368–377
Imada C (2005) Enzyme inhibitors and other bioactive compounds from marine Actinomycetes. Antonie Van Leeuwenhoek 87:59–63. doi:10.1007/s10482-004-6544-x
Valli S, Suvathi SS, Aysha OS, Nirmala P, Vinoth KP, Reena A (2012) Antimicrobial potential of Actinomycetes species isolated from marine environment. Asian Pac J Trop Biomed 2:469–473. doi:10.1016/S2221-1691(12)60078-1
Solanki R, Khanna M, Lal R (2008) Bioactive compounds from marine Actinomyces. Indian J Microbiol 48:410–431. doi:10.1007/s12088-008-0052-z
Kalia VC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 31:224–245. doi:10.1016/j.biotechadv.2012.10.004
Hughes CC, Prieto-Davo A, Jensen PR, Fenical W (2008) The marinopyrroles, antibiotics of an unprecedented structure class from a marine Streptomyces sp. Org Lett 10:629–631. doi:10.1021/ol702952n
Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W (2003) Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew Chem Int Ed Engl 42:355–357. doi:10.1002/anie.200390115
Jensen PR, Gontang E, Mafnas C, Mincer TJ, Fenical W (2005) Culturable marine Actinomycete diversity from tropical Pacific Ocean sediments. Environ Microbiol 7:1039–1048. doi:10.1111/j.1462-2920.2005.00785.x
Riedlinger J, Reicke A, Zahner H, Krismer B, Bull AT, Maldonado LA, Ward AC, Goodfellow M, Bister B, Bischoff D, Sussmuth RD, Fiedler HP (2004) Abyssomicins, inhibitors of the para-aminobenzoic acid pathway produced by the marine Verrucosispora strain AB-18-032. J Antibiot 57:271–279. doi:10.1038/ja.2007.54
Koul S, Jyotsana P, Anjali M, Kalia VC (2016) Potential emergence of multi-quorum sensing inhibitor resistant (MQSIR) bacteria. Indian J Microbiol 56:1–18. doi:10.1007/s12088-015-0558-0
Mukherjee K, Tribedi P, Mukhopadhyay B, Sil AK (2013) Antibacterial activity of long-chain fatty alcohols against Mycobacteria. FEMS Microbiol Lett 338:177–183. doi:10.1111/1574-6968.12043
Kabelitz N, Santos PM, Heipieper HJ (2003) Effect of aliphatic alcohols on growth and degree of saturation of membrane lipids in Acinetobacter calcoaceticus. FEMS Microbiol Lett 220:223–227. doi:10.1016/S0378-1097(03)00103-4
Willis AT (2014) Anaerobic bacteriology: clinical and laboratory practice. Butterworth-Heinemann, London
Rajalakshmi S, Mahesh N (2014) Production and characterization of bioactive metabolites isolated from Aspergillus terreus in rhizosphere soil of medicinal plants. Int J Curr Microbiol Appl Sci 3:784–798
Kalpana Devi V, Shanmugasundaram R, Mohan VR (2012) GC–MS analysis of ethanol extracts of Entada pursaetha dc seed. Biosci Discover 3:30–33
Yogeswari S, Ramalakshmi S, Neelavathy R, Muthumary J (2012) Identification and comparative studies of different volatile fractions from Monochaetia kansensis by GCMS. Glob J Pharmacol 6:65–71
Chaudhary R, Tripathy A (2015) Isolation and identification of bioactive compounds from Irpex Lacteus Wild Fleshy Fungi. J Pharm Sci Res 7:424–434
Salem MZ, Ali HM, Mansour MM (2014) Fatty acid methyl esters from air-dried wood, bark, and leaves of Brachychiton diversifolius R. Br: antibacterial, antifungal, and antioxidant activities. Bioresources 9:3835–3845
Suresh A, Praveenkumar R, Thangaraj R, Oscar FL, Baldev E, Dhanasekaran D, Thajuddin N (2014) Microalgal fatty acid methyl ester a new source of bioactive compounds with antimicrobial activity. Asian Pac J Trop Dis 4:979–984. doi:10.1016/S2222-1808(14)60769-6
Boussaada O, Ammar S, Saidana D, Chriaa J, Chraif I, Daami M, Helal AN, Mighri Z (2008) Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbiol Res 163:87–95. doi:10.1080/10412905.2009.9700142
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253. doi:10.1016/j.ijfoodmicro.2004.03.022
Naoko T, Akiko S, Miki N, Keisuke M, Kazutoyo E, Hajime H, Yoshihiro I (2007) Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecule 12:139–148. doi:10.3390/12020139
Chandrasekar T, Rao MRK, Kumar RV, Prabhu K, NandhaKumar V, Divya D (2015) GC–MS analysis, antimicrobial, antioxidant activity of an Ayurvedic medicine. Nimbapatradi Choornam. J Chem Pharm Res 7:124–136. doi:10.1155/2014/694934
Rahbar N, Shafagha A, Salimi F (2012) Antimicrobial activity and constituents of the hexane extracts from leaf and stem of Origanum vulgare L. sp. Viride (Boiss.) Hayek. Growing wild in Northwest Iran. J Med Plants Res 6:2681–2685. doi:10.5897/JMPR11.1768
Raj Kumar K, Brindha Priyadarisini V, Ranjith Kumar M (2012) Isolation and identification of bioactive compounds from Bacillus Megateriume5 from the south east coastal region of India against urinary tract infectious pathogens. Int J Phar Biol Archives 3:842–847
Usha Nandhini S, Sangareshwari S, Lata K (2015) Gas chromatography-mass spectrometry analysis of bioactive constituents from the marine Streptomyces. Asian J Pharm Clin Res 8:244–246
Girija S, Veeramuthu D, Pandi Suba K, Hariprasad G, Raghuraman R (2014) Chromatographic characterization and GC-MS evaluation of the bioactive constituents with antimicrobial potential from the pigmented ink of Loligo duvauceli. Int Sch Res Notices. doi:10.1155/2014/820745
Al-Youssef HM, Hassan WHB (2015) Antimicrobial and antioxidant activities of Parkinsonia aculeata and chemical composition of their essential oils. Merit Res J Med Med Sci 3:147–157
Olubunmi A, Gabriel OA, Stephen AO, Scott FO (2011) Antioxidant and antimicrobial activity of cuticular wax from Kigelia Africana. FABAD J Pharm Sci 34:187–194
Garaniya N, Bapodra A (2014) Ethno botanical and Phytophrmacological potential of Abrus precatorius L.: a review. Asian Pac J Trop Biomed 4:27–34. doi:10.12980/APJTB.4.2014C1069
Al-Abd NM, Mohamed ZN, Mansor M, Azhar F, Hasan MS, Kassim M (2015) Antioxidant, antibacterial activity, and phytochemical characterization of Melaleuca cajuputi extract. BMC Complement Altern Med 15:1–13. doi:10.1186/s12906-015-0914-y
Nahar N, Rahman S, Rahman SM, Moniruzzaman M (2016) GC-MS analysis and antibacterial activity of Trigonella foenumgraecum against bacterial pathogens. Free Radical Antioxid 6:109–114. doi:10.5530/fra.2016.1.13
Hussain AZ, Kumaresan S (2014) GC-MS studies and phytochemical screening of Sesbania grandiflora L. J Chem Pharm Res 6:43–47
Afolayan AJ, Ashafa AOT (2009) Chemical composition and antimicrobial activity of the essential oil from Chrysocoma ciliata L. leaves. J Med Plant Res 3:390–394
Kuppuswamy MK, Jonnalagadda B, Arockiasamy S (2013) GC-MS analysis of chloroform extract of Croton bonplandianum. Int J Pharm Bio Sci 4:613–617
Bhardwaj A, Shakil NA, Jha V, Gupta RK (2014) Screening of nutritional, phytochemical, antioxidant and antibacterial activity of underutilized seeds of Scirpus articulatus: the basis of Khubahi Ramdana industry. J Pharma Phytochem 3:311–320
Sivakumar SR (2014) GC-MS analysis and antibacterial potential of white crystalline solid from red algae Portieria hornemannii against the plant pathogenic bacteria Xanthomnas axonopodis pv. citri (Hasse) Vauterin et al. and Xanthomonas campestris pv. malvacearum (smith 1901) dye 1978b. Int J of Adv Res 2:174–183
Acknowledgments
The author I. Faridha Begum, Senior research Fellow is grateful for the financial assistance by University Grant Commision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faridha Begum, I., Mohankumar, R., Jeevan, M. et al. GC–MS Analysis of Bio-active Molecules Derived from Paracoccus pantotrophus FMR19 and the Antimicrobial Activity Against Bacterial Pathogens and MDROs. Indian J Microbiol 56, 426–432 (2016). https://doi.org/10.1007/s12088-016-0609-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-016-0609-1