Abstract
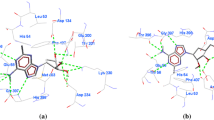
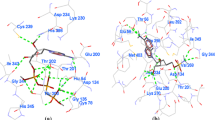
S-adenosyl-L-homocysteine hydrolase of Plasmodium falciparum (PfSAHH) is a potential drug target against malaria, and selective inhibition of PfSAHH is the excellent strategy to prevent the growth of parasite inside the host. Therefore, a comparative analysis of human S-adenosyl-L-homocysteine hydrolase (HsSAHH) and PfSAHH has been performed to explore the structural differences. Structural superimposition of PfSAHH and HsSAHH has generated the RMSD of 0.749 Å over 394 alpha carbon pairs. Residues of PfSAHH from position Tyr152 to Lys193 aligned with insertion/deletion region in HsSAHH, and these extra residues results in an extent of variation in cavity region of PfSAHH. Nicotinamide adenine dinucleotide (NAD) was observed to form hydrogen bonding with Thr201, Thr202, Thr203, Asn235, Val268, Glu287, Asn322, Ile343, Asn391, Lys473, and Tyr477 and also forms hydrophobic interactions with Val268, Ile288, and Thr320 of PfSAHH. In comparison to HsSAHH, Asn322, Lys473, and Tyr477 residues of PfSAHH are unique in interaction with NAD. 2-Fluoroaristeromycin and other analogues of aristeromycin have shown the good binding affinity for both enzymes. Structural differences between PfSAHH and HsSAHH might be employed to design the potential inhibitor of PfSAHH. To find the target enzyme responsible for an anti-malarial effect, molecular docking and interaction analysis of curcumin were performed with 34 drug targets of P. falciparum. Curcumin shows high affinity for binding with HGPRT of PfHGPRT, and an anti-malarial effect of curcumin might be due to binding with PfHGPRT.








Similar content being viewed by others
References
Yuan CS, Saso Y, Lazarides E, Borchardt RT, Robins MJ (1999) Recent advances in S-adenosyl-L-homocysteine hydrolase inhibitors and their potential clinical applications. Expert Opin Ther Patents 9:1197–1206
Kitade Y, Kozaki A, Miwa T, Nakanishi M, Yatome C (2000) Synthesis of carbocyclic nucleosides and their SAH hydrolase inhibitory activities. Nucleic Acids Symp Ser 44:111–112
Tanaka N, Nakanishi M, Kusakabe Y, Shiraiwa K, Yabe S, Ito Y, Kitade Y, Nakamura KT (2004) Three-dimensional structure of S-adenosyl-L-homocysteine hydrolase from Plasmodium falciparum. Nucleic Acids Symp Ser (Oxf) 48:281–282
Tanaka N, Umeda T, Kusakabe Y, Nakanishi M, Kitade Y, Nakamura KT (2013) Structural biology for developing antimalarial compounds. Yakugaku Zasshi 133:527–537
Elrod P, Zhang J, Yang X, Yin D, Hu Y, Borchardt RT, Schowen RL (2002) Contributions of active site residues to the partial and overall catalytic activities of human S-adenosylhomocysteine hydrolase. Biochemistry 41:8134–8142
Kojima H, Yamaguchi T, Kozaki A, Nakanishi M, Ueno Y, Kitade Y (2002) Synthesis of noraristeromycin analogues possessing SAH hydrolase inhibitory activity for the development of antimalaria agents. Nucleic Acids Res Suppl 2:141–142
Kitade Y, Kojima H, Zulfiqur F, Kim HS, Wataya Y (2003) Synthesis of 2-fluoronoraristeromycin and its inhibitory activity against Plasmodium falciparum S-adenosyl-L-homocysteine hydrolase. Bioorg Med Chem Lett 13:3963–3965
Nakanishi M (2007) S-adenosyl-L-homocysteine hydrolase as an attractive target for antimicrobial drugs. Yakugaku Zasshi 127:977–982
Bujnicki JM, Prigge ST, Caridha D, Chiang PK (2003) Structure, evolution, and inhibitor interaction of S-adenosyl-L-homocysteine hydrolase from Plasmodium falciparum. Proteins 52:624–632
Mimche PN, Taramelli D, Vivas L (2011) The plant-based immunomodulator curcumin as a potential candidate for the development of an adjunctive therapy for cerebral malaria. Malar J 15(10 Suppl 1):S10
Singh DB, (2014) Success, limitation and future of computer aided drug designing translational medicine doi:10.4172/2161-1025.1000e127
Singh DB, Gupta MK, Singh DV, Singh SK, Misra K (2013) Docking and in silico ADMET studies of noraristeromycin, curcumin and its derivatives with Plasmodium falciparum SAH hydrolase: a molecular drug target against malaria. Interdiscip Sci 5:1–12
Ando T, Iwata M, Zulfiqar F, Miyamoto T, Nakanishi M, Kitade Y (2008) Synthesis of 2-modified aristeromycins and their analogs as potent inhibitors against Plasmodium falciparum S-adenosyl-L-homocysteine hydrolase. Bioorg Med Chem 16:3809–3815
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Stierand K, Rarey M (2010) Drawing the PDB: protein-ligand complexes in two dimensions. ACS Med Chem Lett 1:540–545
Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27:343–350
Thomsen R, Christensen MH (2006) MolDock: a new technique for high-accuracy molecular docking. J Med Chem 49:3315–3321
Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu G, Lee PW, Tang Y (2012) admetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties. J Chem Inf Model 52:3099–3105
Matiugina ES, Seley-Radtke KL, Andronova VL, Galegov GA, Kochetkov SN, Khandazhinskaia AL (2010) Synthesis and antiviral evaluation against Vaccinia virus of new N1-oxide analogues of 5′-noraristeromycin. Bioorg Khim 36:797–801
Das SR, Schneller SW, Balzarini J, De Clercq E (2002) A mercapto analogue of 5′-noraristeromycin. Bioorg Med Chem 10:457–460
Seley KL, Schneller SW, Rattendi D, Bacchi CJ (1997) (+)-7-Deaza-5′-noraristeromycin as an anti-trypanosomal agent. J Med Chem 40(4):622–624
Aarbakke J, Miura GA, Prytz PS, Bessesen A, Slørdal L, Gordon RK, Chiang PK (1986) Induction of HL-60 cell differentiation by 3-deaza-(+/−)-aristeromycin, an inhibitor of S-adenosylhomocysteine hydrolase. Cancer Res 46:5469–5472
Suksangpleng T, Leartsakulpanich U, Moonsom S, Siribal S, Boonyuen U, Wright GE, Chavalitshewinkoon-Petmitr P (2014) Molecular characterization of Plasmodium falciparum uracil-DNA glycosylase and its potential as a new anti-malarial drug target. Malar 13:149
Tagboto S, Townson S (2001) Antiparasitic properties of medicinal plants and other naturally occurring products. Adv Parasitol 50:199–295
Rasoanaivo P, Wright CW, Willcox ML, Gilbert B (2011) Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malar J 15(10 Suppl 1):S4
Raman J, Ashok CS, Subbayya SI, Anand RP, Selvi ST, Balaram H (2005) Plasmodium falciparum hypoxanthine guanine phosphoribosyltransferase. Stability studies on the product-activated enzyme. FEBS J 272:1900–1911
Queen SA, Vander JDL, Reyes P (1989) Characterization of adenine phosphoribosyltransferase from the human malaria parasite, Plasmodium falciparum. Biochim Biophys Acta 996:160–165
Burger PB, Williams M, Sprenger J, Reeksting SB, Botha M, Müller IB, Joubert F, Birkholtz LM, Louw AI (2015) A novel inhibitor of Plasmodium falciparum spermidine synthase: a twist in the tail. Malar J 14:54
Raman J, Mehrotra S, Anand RP, Balaram H (2004) Unique kinetic mechanism of Plasmodium falciparum adenylosuccinate synthetase. Mol Biochem Parasitol 138:1–8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(GIF 136 kb)
Rights and permissions
About this article
Cite this article
Singh, D.B., Dwivedi, S. Structural insight into binding mode of inhibitor with SAHH of Plasmodium and human: interaction of curcumin with anti-malarial drug targets. J Chem Biol 9, 107–120 (2016). https://doi.org/10.1007/s12154-016-0155-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12154-016-0155-7