Abstract
The effluent from a continuous-flow stirred tank reactor (CSTR) treating synthetic wastewater was used as an alternative carbon source to glucose for algal biomass and biodiesel production. The influence of the effluent on microalgal cell growth and lipid yield, as well as the utilization of volatile fatty acids (VFAs) in the effluent were investigated. The results indicate that C. protothecoides can proliferate in the CSTR effluent and accumulate biolipid. The final lipid content of the culture with the effluent feeding was 27 ± 1.11 % after 168 h cultivation in flasks, which was higher than that with glucose of the same COD concentration. Valeric acid, ethanol, and butyric acid were favorable carbon sources for cell growth. The soluble microbial products (SMP) can be used as a carbon source for cell growth, and the existence of SMP could protect the cell from the inhibition caused by strong VFAs and improve the utilization of VFAs.
Similar content being viewed by others
Introduction
Due to the rate of depletion of fossil fuels and the effect of greenhouse gas emissions on global climate change, production of alternative and more sustainable fuel materials has attracted great attention [1]. Biodiesel, one of the most commonly used biofuels, is recognized as an ideal sustainable energy source, and thus as a possible primary energy source in the future [2]. The raw materials used for biodiesel production are currently produced from oil producing plants such as palm trees, soybeans, jatropha, and rapeseeds [3]. Other sources include animal fat and waste cooking oil [4, 5]. On October 28, 2011, biofuels were used to achieve the first test flight by Air China Limited. The biological material used for this test was from jatropha seeds. Although this test increased confidence in the biofuels market, experts pointed out that the price of the biofuel was 1.5 to 2 times that of traditional fuel [6].
An inadequate source of raw materials makes large-scale production of biodiesel difficult, and it is the biggest obstacle for the biodiesel production industry. Microalgae have been widely recognized as a promising candidate for fuel production because of their extremely rapid growth rate, high biomass production and high oil production capacity compared to other energy crops [2]. Phototrophic cultivation is the most frequently used cultivation method for production of microalgae in the biodiesel production attempts, however, the oil productivity of this approach is typically lower than that of heterotrophic cultivation, due mainly to slow cell growth and low biomass production [7]. With heterotrophic algae, the specific surface area of the reactor could be reduced since the algae do not need any light for growing. Furthermore, the organic composition of the algae can be influenced by using the appropriate culture medium [8]. Chlorella protothecoides is the most studied algae strain for heterotrophic cultivation [9–11]. Xiong et al. [11] indicated that the maximum lipid content for the microalgae was 57.8 % under an improved fed-batch culture strategy. Although high oil productivity could be achieved, contamination of the culture medium and the high costs of an organic carbon source remain major concerns for commercial production.
Great research effort has been undertaken to reduce the cost of heterotrophic biodiesel production from microalgae. Recent research has considered the possibility of using wastewater as a feedstock for biodiesel. Cheng et al. [12] used hydrolysate of Jerusalem artichoke tuber as carbon source to accumulate a 44 % (dry biomass) of lipid content using heterotrophic C. protothecoides. However, Jerusalem artichoke is not generally considered as a waste product. In further research, anaerobically digested dairy manure [13] and wastewater containing 85–90 % carpet mill effluents [14] were used as a carbon source for production of lipids for biofuel, however the lipid content was relatively low at 13.7 and 6.82 %, respectively. In this research, we attempted to use the effluent from a stably operated anaerobic continuous-flow stirred tank reactor (CSTR) treating synthetic wastewater as an alternative carbon source to glucose for the cultivation of C. protothecoides, and examine the accumulation of lipid content. The result proved that C. protothecoides can use the small molecular volatile fatty acids (VFAs) in the effluent as the carbon source for cell growth and lipid accumulation. The research provides a mode of microalgae cultivation, i.e., effluent-based cultivation, a combination process unites the microalgae biodiesel production with the wastewater treatment. The goal is to reduce the biodiesel cost and further increase the lipid yield.
Material and Methods
Microalgal Strain and Media
C. protothecoides FACHB-3, obtained from the Freshwater Algae Culture Collection of the Institute of Hydrobiology (FACHB), Chinese Academy of Sciences (Wuhan, China), was selected as the unicellular green microalgae in this research.
The culture media was a modified selenite enrichment medium containing the following: NaNO3 250 mg/l, KH2PO4 70 mg/l, K2HPO4 30 mg/l, MgSO4·7H2O 30 mg/l, FeSO4·7H2O 0.3 mg/l, H3BO3 2.86 mg/l, MnCl2·4H2O 1.81 mg/l, ZnCl2 0.105 mg/l, Na2MoO4·2H2O 0.39 mg/l, CuSO4·5H2O 0.08 mg/l, and CoCl2 0.030 mg/l. Glucose at concentrations of 5 and 15 g/l, CSTR effluent, glucose (5 g/l) combined with effluent and commercial VFAs with different concentrations were added into the basal medium according to the experimental design. The compositions of the carbon sources in the media used in different experiment stages are listed in Table 1.
Characteristics of Effluent from the Anaerobic CSTR
An anaerobic CSTR, with a total volume of 20 l, was used to treat synthetic organic wastewater, and the effluent was used as the carbon source for the microalgae cultivation. The synthetic wastewater containing sugarcane 6 g/l, NH4Cl 136.4 mg/l, KH2PO4 30 mg/l and MgSO4·7H2O 30 mg/l, with the C/N/P ratio of 1,000:5:1, was fed to the CSTR. A peristaltic pump provided a 35 ml/min continuous inflow to give a hydraulic retention time of 9.5 h. The operating temperature was kept at 35 °C. The CSTR was operated in a balanced mode with a constant chemical oxygen demand (COD) and a stable organic composition in the effluent. The effluent was then filtered through a hollow fiber membrane with molecular weight cut-off of 20,000 and neutralized to pH 6.8 by 1 mol/l NaOH. The COD and the organic compositions of the effluent are shown in Table 1. The average COD of the effluent was 5300 mg/l. In addition to VFAs, the effluent contained sugar, protein and other elements in the form of soluble microbial products (SMP). Little phosphorus and nitrogen was detected in the effluent.
Sterilization of the CSTR Effluent
The effluent from the CSTR was sterilized before use, and three sterilization processes, including steam sterilization, 0.22 μm microfiltration and UV irradiation were used in the experiment. The effect of the different sterilization processes on the cultivation was investigated.
The right amount of trace elements was added to the effluent before sterilization. For steam sterilization, the media was sealed and sterilized at 121 °C and 120 kPa for 30 min. For microfiltration, the media was filtered through a 0.22 μm microfilter. For UV irradiation, the media was exposed to UV light with a 254 nm wavelength for 15 min.
Microalgae Cultivation in Flasks
Heterotrophic cultivation was carried out in 500 ml Erlenmeyer flasks containing 200 ml of the culture medium at a temperature of 28 °C with continuous shaking at 160 rpm. A 50-ml air flow was supplied to each flask for one hour per day. The flasks were kept in darkness during cultivation. The culture media that was supplemented with 15 and 5 g/l glucose were used as control to evaluate the effect of the effluent on C. protothecoides growth. The culture was sterilized at 121 °C and 120 kPa for 30 min before use (except as noted in the sterilization experiment). All the cultivations were carried out three times expect as noted and the average values were reported.
Cell Collection and Lipid Extraction
Algal cells were collected by centrifugation at 7,000 rpm for 5 min at each end of the batch cultivation. The cell pellets were then dried in an oven (GZX-9070 MBE, Shanghai, China) at 65 °C to reach a constant weight. Afterwards, dried cell pellets were pulverized in a mortar for 30 min. Lipids was then extracted from the disrupted samples with n-hexane in a Soxhlet extractor. The whole process flow schematic for lipid production for biodiesel from the CSTR effluent is shown in Fig. 1.
Analytical Methods
Cell growth was measured at OD 540 nm [15] using an UV/Visible spectrophotometer (T6 PERSEE, Beijing, China). Glucose concentration was estimated by digestion followed by chemical titration according to Chinese SEPAC Standard Methods [16]. Ethanol and VFAs were measured using a gas chromatograph (GC 7890N/FID, Agilent, US) fitted with an HP-INNOWAX 19095N-123 column. The column temperature was 70 °C for the initial 0 min and was increased by 25 °C/min to 170 °C and retained at this level for 2 min. The injector temperature was set at 250 °C and the flame ionization detector was at 300 °C. The lipid content of the microalgal cells was determined using n-hexane/alcohol lipid extraction as described by [17]. The SMP concentration in the CSTR effluent was determined by a total organic carbon (TOC) analyzer with an autosampler (TOC-VCPN, Shimadzu, Japan) after 0.45 μm filtration [18].
Results and Discussion
Sterilization of the CSTR Effluent
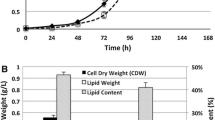
The effluent from the CSTR was sterilized before use to avoid contamination during the cultivation. The media in previous studies used for the heterotrophic cultivation of C. protothecoides were reported to be sterilized with steam at 112 °C [10, 19]. However, a more sustainable sterilization process should be developed that is suited for waste derived carbon source pretreatment. Three sterilization processes were introduced in this study as stated in the “Material and Methods” part. A typical profile of the effect of different sterilization processes on algal cell growth and lipid accumulation is shown in Fig. 2. The growth curves in Fig. 2a show that the cell density of C. protothecoides reached 0.425, 0.325, and 0.325 g/l within 180 h in the culture media pretreated with steam sterilization, 0.22 μm microfiltration and UV irradiation, respectively. The corresponding lipid content for these three sterilization processes was 27.0 ± 1.4 %, 22.3 ± 1.27 %, and 18.5 ± 1.21 %, respectively (Fig. 2b). These data suggest that steam sterilization was the most effective pretreatment process of those used for heterotrophic growth of C. protothecoides. The results also indicate that 0.22 μm microfiltration may be an alternative option when considering the lipid content that was achieved, especially when a commercial scale bioreactor is in use. However, steam sterilization was used in the subsequent experiments.
Comparison of Different Carbon Source on Algal Cultivation
Glucose is the most popular carbon source in heterotrophic C. protothecoides cultivation [9, 20], and 15 g/l glucose in the medium was proved to be the best concentration for heterotrophic C. protothecoides cultivation in our previous study (data not shown here). However, using a hydrolysate of organic wastewater as the carbon source for microalgal biodiesel production was seldom investigated. The total COD concentration of the CSTR effluent in this study was 5300 mg/l on average, which is equivalent to the COD value of 5 g/l glucose. Therefore, basal media containing 5 and 15 g/l glucose was used as control. A combined carbon source (effluent and 5 g/l glucose mixed with equal volume) was also used in the experiment. The experiments were operated under the same cultivation condition.
The typical growth curves in Fig. 3a show that all the four cultures had an exponential growth phase during 15 to 50 h, and that the maximum cell density of C. protothecoides was achieved at 48 h in all cases except for the growth in the CSTR effluent, which was achieved at 168 h. The maximum cell density was 0.48, 0.39, 0.39, and 0.43 g/l, in the culture Glucose 1#, Glucose 2#, Mixed, and CSTR effluent, respectively. The final lipid content of the culture with CSTR effluent was similar to that with Glucose 1# and the Mixed (27 ± 1.11 %, 32 ± 1.91 %, and 29 ± 1.25 %, respectively) and was higher than that in Glucose 2# (20 ± 0.76 %) as shown in Fig. 3b. The average lipid yield of 24, 13, 18, and 17 mg l−1 day−1 was obtained in the culture of Glucose 1#, Glucose 2#, Mixed, and the CSTR effluent, respectively. The result indicates that C. protothecoides can proliferate in the wastewater hydrolysate and accumulate rather high amount of lipid in the cells.
The Utilization of VFAs in the Effluent
Substrates such as sweet sorghum [19], hydrolysate of Jerusalem artichoke tuber [12], anaerobic digested dairy manure [13], and wastewater containing 85-90 % carpet industry effluents [14] were used as an alternative to glucose in previous reported studies. However, these studies did not investigate the mechanism of substrate utilization to reveal the most favorable components in these substrates that were used by C. protothecoides. In this study, the concentrations of the VFAs in the effluent were monitored during the cultivations, typical results are shown in Fig. 4. The curves show that valeric acid, ethanol, and butyric acid, in that order, were consumed rapidly by C. protothecoides for cell growth. Each was completely consumed at approximately 20, 28, and 36 h respectively. The concentration of propionic acid decreased slowly during the cultivation, and it was completely consumed at about 70 h. Acetic acid was present throughout the cultivation. The concentration of acetic acid slightly increased in the first 50 h, then fell abruptly from 460 mg/l to less than 20 mg/l during the following 40 h. Correlated with the typical lipid content profile in Fig. 3b, it can be roughly deduced that all of the valeric acid, butyric acid, ethanol and half of the propionic acid were used by C. protothecoides for cell growth. Half of the propionic acid and all the acetic acid were used by C. protothecoides for other metabolic activities, for example, lipid synthesis. Definite growth of Chlorella due to the presence of the organic acids was reported by Eny [21] in their study on the respiratory mechanism of plant tissues. They found that acetate and butyrate could be assimilated for cell growth, while little propionate was used for growth. The synthesis of storage material may result from directly oxidative assimilation of an organic substrate in heterotrophic growth of Chlorella [22]. It was also reported that acetate was a satisfactory carbon source for the growth of some species of Flagellata in dark conditions [21]. Therefore, further research is recommended to determine whether the assimilation of acetate may direct a lipid metabolism.
Influence of SMP
Microbial-related substances, SMP, occupied 50 % of the total COD concentration of the CSTR effluent (Table 1), which is different from the traditional cultivation substrate. Because of this, the influence of SMP on the cell growth and lipid accumulation was investigated in this study. Three types of substrate were used in this stage, as shown in Table 1. Commercial VFAs were used and mixed in the same ratio with that of VFAs found in the CSTR effluent to simulate the COD value of VFAs in the effluent (in VFAs1#) and the total COD value of the effluent (VFAs 2#). The experiments were operated under the same cultivation condition and each of the cultivation was carried out in duplicate. The typical algal cell growth and average lipid accumulation during the cultivation are shown in Fig. 5.
The dry cell yield reached the maximum value of 0.6 g/l after 156 h cultivation with the substrate from the CSTR effluent, while the maximum value was 0.52 and 0.25 g/l with VFAs 1# (the same COD value with VFAs in the effluent) and VFAs 2# (the same COD value with total COD of the effluent), respectively. Compared with the effluent, the biomass production of VFAs1# was decreased by 13 %. The results indicate that SMP may be used as a carbon source by C. protothecoides for cell growth due to its biodegradability [23]. The biomass production of VFAs 2# was decreased by 58 %, which indicates that the existence of SMP may have protected the microalgal cell from growth inhibition caused by strong VFAs and improved the utilization of VFAs by C. protothecoides for cell growth. A similar phenomenon was observed when SMP was added to an activated sludge system to degrade glucose [24], where the degradation rate was increased with the presence of SMP. The average lipid content in algal cells was 25.37 % with the effluent feeding, and 23.21 and 19.07 % with the feeding from VFAs 1# and VFAs 2#, respectively. The results indicate that not only cell synthesis, but also the lipid yield from the CSTR effluent has exceeded those from the synthetic substrates of VFAs.
Conclusions
Anaerobic hydrolysis has been widely employed to treat organic wastewater. The hydrolysate is a potential carbon source for heterotrophic microalgal biodiesel production. This study established the proof-of-concept for biodiesel production from effluent-based culture. The results indicate that C. protothecoides can proliferate in a wastewater hydrolysate and that the lipid content was higher than that in a glucose solution with the same COD concentration. The utilization of VFAs in the effluent and the influence of SMP on the cultivation were investigated. The results indicate that the recovery energy from heterotrophic algal cultivation with wastewater hydrolysate appears promising for future biofuel applications.
References
Lee JY, Yoo C, Jun SY, Ahn CY, Oh HM (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101:S75–77
Chisti Y (2007) Research review paper: biodiesel from microalgae. Biotechnol Adv 25:294–306
Sharma YC, Singh B (2009) Development of biodiesel: current scenario. Renew Sust Energy Rev 13:1646–1651
Felizardo P, Correia MJN, Raposo I, Mendes JF, Berkemeier R, Bordado JM (2006) Production of biodiesel from waste frying oil. Waste Manage 26:487–94
Kulkarni MG, Dalai AK (2006) Waste cooking oil—an economical source for biodiesel: a review. Ind Eng Chem Res 45:2901–2913
Chen HH, Yi RR (2011) A break-through on aerial biodiesel production industrialization is going to be achieved. http://www.newenergy.org.cn/html/01111/1171143354.html. Accessed 07 November 2011 [in Chinese]
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Kröger M, Müller-Langer F (2011) Impact of heterotrophic and mixotrophic growth of microalgae on the production of future biofuels. Biofuels 2(2):145–151
Miao XL, Wu QY (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846
Li XF, Xu H, Wu QY (2007) Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactor. Biotechnol Bioeng 98:764–771
Xiong W, Li XF, Xiang JY, Wu QY (2008) High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl Microbiol Biotechnol 78:29–36
Cheng Y, Zhou WG, Gao CF, Lan K, Gao Y, Wu QY (2009) Biodiesel production from Jerusalem artichoke (Helianthus Tuberosus L.) tuber by heterotrophic microalgae Chlorella protothecoides. J Chem Technol Biothechnol 84:777–781
Wang L, Li YC, Chen P, Min M, Chen YF, Zhu J, Ruan RR (2010) Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour Technol 101:2623–2628
Chinnasamy S, Bhatnagar A, Hunt RW, Das KC (2010) Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour Technol 101:3097–3105
Becker EW (1994) Measurement of algal growth. In: Microalgae: Biotechnology and Microbiology, Cambridge University Press, Cambridge, pp 56–62
Chinese SEPAC (ed) (1997) Standard methods for the examination of water and wastewaters, 3rd edn. State Environmental Protection Association of China, Beijing
Halim R, Gladman B, Danquah MK, Webley PA (2010) Oil extraction from microalgae for biodiesel production. Bioresour Technol 102:178–185
Carlson KH, Amy GL (2000) The importance of soluble microbial products (SMPs) in biological treatment. Water Res 34:1386–1396
Gao CF, Zhai Y, Ding Y, Wu QY (2010) Application of sweet sorghum for biodiesel production by heterotrophic microalga Chlorella protothecoides. Appl Energy 87:756–761
Wu QY, Yin S, Sheng GY, Fu JM (1992) A comparative study of gases generated from stimulant thermal degradation of autotrophic and heterotrophic Chlorella. Progr Nat Sci 2:311–318
Eny DM (1950) Respiration studies on Chlorella. I. Growth experiments with acid intermediates. Plant Physiol 25:478–495
Myers J, Cramer LM (1947) Reconsideration of the photo synthetic mechanism in Chlorella. Science 105:552
Laspidou CS, Rittmann BE (2002) A unified theory for extracellular polymeric substances soluble microbial products, and active and inert biomass. Water Res 36:2711–2720
Zhang B, Yamamoto K (1996) Seasonal change of microbial population and activities in a building wastewater reuse system sing a membrane separation activated sludge process. Water Sci Technol 34:295–302
Acknowledgments
We gratefully acknowledge the financial support provided by the State Key Laboratory for Urban Water Resources and Environment (Harbin Institute of Technology) (2012TS02) and the National Innovation Team supported by the National Science Foundation of China (No.50821002). The authors acknowledge the language help from Dr Baden Myers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wen, Q., Chen, Z., Li, P. et al. Lipid Production for Biofuels from Effluent-based Culture by Heterotrophic Chlorella Protothecoides . Bioenerg. Res. 6, 877–882 (2013). https://doi.org/10.1007/s12155-013-9308-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-013-9308-5