Abstract
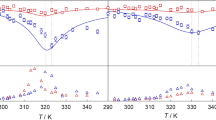
This paper is addressing the challenge related to understanding the experimental results obtained by Taylor dispersion technique when working under high pressure with supercritical carbon dioxide (CO2). We discuss typical experimental problems that arise when using the Taylor dispersion method for measuring diffusion coefficients in the supercritical fluids. The diffusion coefficients of the two samples in supercritical carbon dioxide were measured along the isobars at different temperatures. The diffusion coefficients of ethanol in supercritical CO2 have been measured in the temperature range from 304.15 to 343 K along the isobar p = 12.0 MPa. Experiments with the gaseous CH4/CO2 mixture with a mole fraction of CH4 = 0.2052 mol mol− 1 have been conducted in the temperature range from 317.85K to 343.15 K along the isobar p = 12.5 MPa. A comparative analysis of a diffusion behavior of these very different mixtures is presented.










Similar content being viewed by others
References
Alizadeh, A., Nieto de Castro, C.A., Wakeham, W.A.: The theory of the taylor dispersion technique for liquid diffusivitymeasurements. Int. J. Thermophys. 1(3), 243–284 (1980)
Ancherbak, S., Santos, C., Legros, J.C., Mialdun, A., Shevtsova, V.: Development of a high-pressure set-up for measurements of binary diffusion coefficients in supercritical carbon dioxide. Eur. Phys. J E 39(11), 111 (2016)
Aris, R.: On the dispersion of a solute in a fluid flowing through a tube. Proc. R. Soc. London A 235, 67–77 (1956)
Bou-Ali, M.M., Ahadi, A., Alonso de Mezquia, D., Galand, Q., Gebhardt, M., Khlybov, O., Köhler, W., Larrañaga, M., Legros, J.C., Lyubimova, T., Mialdun, A., Ryzhkov, I., Saghir, M.Z., Shevtsova, V., Van Vaerenbergh, S.: Benchmark values for the soret, thermodiffusion and molecular diffusion coefficients of the ternary mixture tetralin+isobutylbenzene+n-dodecane with 0.8-0.1-0.1 mass fraction. Eur. Phys. J. E 38(4), 30 (2015)
Cadogan, S.P., Maitland, G.C., Trusler, J.P.M.: Diffusion coefficients of co2 and n2 in water at temperatures between 298.15 k and 423.15 k at pressures up to 45 mpa. J. Chem. Eng. Data. 59(2), 519–525 (2014)
Erdogan, M.E., Chatwin, P.: The effects of curvature and buoyancy on the laminar dispersion ofsolute in a horizontal tube. J. Fluid Mech. 29, 3465–484 (1967)
Firoozabadi, A., Myint, P.C.: Prospects for subsurface CO2 sequestration. AIChE J 56, 1398–1405 (2010)
Funazukuri, T., Yi Kong, C., Kagei, S.: Binary diffusion coefficients in supercritical fluids. recent progress in measurements and correlations for binary diffusion coefficients. J. Supercrit. Fluids 38(2), 201–210 (2006)
Guevara-Carrion, G., Ancherbak, S., Mialdun, A., Vrabec, J., Shevtsova, V.: Diffusion of methane in supercritical carbon dioxide across the widomline. Sci. Rep. 9, 8466 (2019)
Han, S.: Anomalous change in the dynamic of a supercritical fluid. Phys. Rev. E 84, 051204 (2011)
Leaist, D.G.: Ternary diffusion coefficients of 18-crown-6 ether-kcl-water by direct least-squares analysis of taylor dispersion measurements. J .Chem. Soc. Faraday Trans. 87, 597–601 (1991)
Leaist, D.G.: Determination of ternary diffusion coefficients by the Taylordispersion method. J. Phys. Chem. 94, 5180–5183 (2019)
Legros, J.C., Mialdun, A., Strizhak, P., Shevtsova, V.: Permeation of supercritical co2 through perfluoroelastomers. J. Supercrit. Fluids 126, 1–13 (2017)
Lemmon, E.W., Huber, M.L., McLinden, M.O.: NIST standard reference database 23: Reference fluid thermodynamic and transport properties-REFPROP, V ersion 9.1 (2013)
Levelt Sengers, J.M.H., Deiters, U.K., Klask, U., Swidersky, P, Schneider, G.M.: Application of the taylor dispersion method in supercritical fluids. Int. J Thermophys 14(4), 893–922 (1993)
Lin, R., Tavlarides, L.L.: Diffusion coefficients of diesel fuel and surrogate compounds in supercritical carbon dioxide. J. Supercrit. Fluids 52(1), 47–55 (2010)
Mialdun, A., Shevtsova, V.: Open questions on reliable measurements of Soret coefficients. Microgravity Sci. Technol. 21, 31–36 (2009)
Mialdun, A., Sechenyh, V., Legros, J.C., Ortiz de Zrate, J.M., Shevtsova, V.: Investigation of fickian diffusion in the ternary mixture of 1,2,3,4-tetrahydronaphthalene, isobutylbenzene, and dodecane. J. Chem. Phys. 139(10), 104903 (2013)
Mialdun, A., Ryzhkov, I., Khlybov, O., Lyubimova, T., Shevtsova, V.: Measurement of Soret coefficients in a ternary mixture of toluene-methanol-cyclohexane in convection-free environment. J. Chem. Phys. 148(4), 044506 (2018)
Nishiumi, H., Kubota, T.: Tracer diffusion coefficients of benzene in dense co2 at 313.2 Kand 8.5-30 MPa. Fluid Phase Equilib. 261, 146–151 (2007)
Nunge, R., Lin, T., Gill, W.: Laminar dispersion in curved tubes and channels. J. Fluid Mech. 51, 363–383 (1972)
Santos, C.I.A.V., Mialdun, A., Legros, J.C., Shevtsova, V.: Sorption equilibria and diffusion of toluene, methanol, and cyclohexane in/through elastomers. J. Applied Polymer Science 133(21), 43449 (2016)
Secuianu, C., Maitland, G.C., Trusler, J.P.M., Wakeham, W.A.: Mutual diffusion coefficients of aqueous kcl at high pressures measured by the taylor dispersion method. J. Chem. Eng. Data. 56(12), 4840–4848 (2011)
Taylor, G.: Dispersion of soluble matter in solvent flowing slowly through atube. Proc. R. Soc. London A 219 (1137), 186–203 (1953)
Taylor, G.: Conditions under which dispersion of a solute in a stream of solvent can be used to measure molecular diffusion. Proc. R. Soc. London A 225(1163), 473–477 (1954)
Thibeau, S., Chiquet, P., Prinet, C., Lescanne, M.: Lacq-Rousse CO2 capture and storage demonstration pilot: lessons learned from reservoir modelling studies. Energy Procedia 37, 6306–6316 (2013)
Triller, T., Bataller, H., Bou-Ali, M.M., Braibanti, M., Croccolo, F., Ezquerro, J.M., Galand, Q., Gavaldá, Jna, Lapeira, E., Laverón-Simavilla, A., Lyubimova, T., Mialdun, A., Ortiz de Zárate, J.M., Rodríguez, J., Ruiz, X., Ryzhkov, I.I., Shevtsova, V., Van Vaerenbergh, S., Köhler, W.: Thermodiffusion in ternary mixtures of water/Ethanol/Triethylene glycol: First report on the DCMIX3-Experiments performed on the international space station. Microgravity Sci. Technol. 30(3), 295–308 (2018)
Umezawa, S., Nagashima, A.: Measurement of the diffusion coefficients of acetone, benzene, and alkane in supercritical co2 by the taylor dispersion method. J. Supercrit. Fluids 5, 242–250 (1992)
Van Vaerenbergh, S., Legros, J.-C., Dupin, J.-C.: First results of soret coefficient measurement experiment. Adv. Space Res. 16(8), 69–81 (1995). EURECA Scientific Results
Van Vaerenbergh, S., Garandet, J.P., Praizey, J.P., Legros, J.C.: Reference soret coefficients of natural isotopes and diluted alloys of tin. Phys. Rev. E 58, 1866–1873 (1998a)
Van Vaerenbergh, S., Legros, J.C.: Soret coefficients of organic solutions measured in the microgravity SCM experiment and by the flow and bénard cells. J. Phys. Chem. B 102(22), 4426–4431 (1998b)
Van Vaerenbergh, S., Srinivasan, S., Saghir, M.Z.: Thermodiffusion in multicomponent hydrocarbon mixtures: Experimental investigations and computational analysis. J. Chem. Phys. 131(11), 114505 (2009)
Yi Kong, C., Funazukuri, T., Kagei, S.: Binary diffusion coefficients and retention factors for polar compounds in supercritical carbon dioxide by chromatographic impulse response method. J. Supercrit. Fluids 37(3), 359–366 (2006)
Acknowledgements
YG, VG and VS acknowledge support by the PRODEX program of the Belgian Federal Science Policy Office. CIAVS is grateful for the funding granted by FEDER –European Regional Development Fund through the COMPETE Programme and FCT -Fundacao para a Ciencia e a Tecnologia, for the KIDIMIX project POCI-01-0145-FEDER-030271.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Thirty Years of Microgravity Research - A Topical Collection Dedicated to J. C. Legros
Guest Editor: Valentina Shevtsova
Rights and permissions
About this article
Cite this article
Gaponenko, Y., Gousselnikov, V., Santos, C.I.A.V. et al. Near-Critical Behavior of Fick Diffusion Coefficient in Taylor Dispersion Experiments. Microgravity Sci. Technol. 31, 475–486 (2019). https://doi.org/10.1007/s12217-019-09736-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12217-019-09736-4