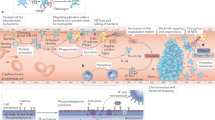
Abstract
The coagulation protease cascade plays an essential role in hemostasis. In addition, a clot contributes to host defense by limiting the spread of pathogens. Coagulation proteases induce intracellular signaling by cleavage of cell surface receptors called protease-activated receptors (PARs). These receptors allow cells to sense changes in the extracellular environment, such as infection. Viruses activate the coagulation cascade by inducing tissue factor expression and by disrupting the endothelium. Virus infection of the heart can cause myocarditis, cardiac remodeling, and heart failure. A recent study using a mouse model have shown that tissue factor, thrombin, and PAR-1 signaling all positively regulate the innate immune during viral myocarditis. In contrast, PAR-2 signaling was found to inhibit interferon-β expression and the innate immune response. These observations suggest that anticoagulants may impair the innate immune response to viral infection and that inhibition of PAR-2 may be a new strategy to reduce viral myocarditis.

Similar content being viewed by others
References
Shauer, A., Gotsman, I., Keren, A., Zwas, D. R., Hellman, Y., Durst, R., et al. (2013). Acute viral myocarditis: current concepts in diagnosis and treatment. The Israel Medical Association journal : IMAJ, 15(3), 180–185.
Schultheiss, H. P., Kuhl, U., & Cooper, L. T. (2011). The management of myocarditis. European heart journal, 32(21), 2616–2625. doi:10.1093/eurheartj/ehr165.
Liu, P. P., & Mason, J. W. (2001). Advances in the understanding of myocarditis. Circulation, 104(9), 1076–1082.
Antoniak, S., Boltzen, U., Riad, A., Kallwellis-Opara, A., Rohde, M., Dorner, A., et al. (2008). Viral myocarditis and coagulopathy: increased tissue factor expression and plasma thrombogenicity. Journal of molecular and cellular cardiology, 45(1), 118–126. doi:10.1016/j.yjmcc.2008.03.013.
Imazio, M., & Cooper, L. T. (2013). Management of myopericarditis. Expert review of cardiovascular therapy, 11(2), 193–201. doi:10.1586/erc.12.184.
Blauwet, L. A., & Cooper, L. T. (2010). Myocarditis. Progress in cardiovascular diseases, 52(4), 274–288. doi:10.1016/j.pcad.2009.11.006.
Thompson, J. M., & Iwasaki, A. (2008). Toll-like receptors regulation of viral infection and disease. Advanced drug delivery reviews, 60(7), 786–794. doi:10.1016/j.addr.2007.11.003.
Kemball, C. C., Alirezaei, M., & Whitton, J. L. (2010). Type B coxsackieviruses and their interactions with the innate and adaptive immune systems. Future microbiology, 5(9), 1329–1347. doi:10.2217/fmb.10.101.
Huber, S. (2008). Host immune responses to coxsackievirus B3. Current topics in microbiology and immunology, 323, 199–221.
Yajima, T. (2011). Viral myocarditis: potential defense mechanisms within the cardiomyocyte against virus infection. Future microbiology, 6(5), 551–566. doi:10.2217/fmb.11.40.
Leipner, C., Grun, K., Schneider, I., Gluck, B., Sigusch, H. H., & Stelzner, A. (2004). Coxsackievirus B3-induced myocarditis: differences in the immune response of C57BL/6 and Balb/c mice. Medical microbiology and immunology, 193(2–3), 141–147. doi:10.1007/s00430-003-0199-5.
Weinzierl, A. O., Szalay, G., Wolburg, H., Sauter, M., Rammensee, H. G., Kandolf, R., et al. (2008). Effective chemokine secretion by dendritic cells and expansion of cross-presenting CD4-/CD8+ dendritic cells define a protective phenotype in the mouse model of coxsackievirus myocarditis. Journal of virology, 82(16), 8149–8160. doi:10.1128/JVI.00047-08.
Negishi, H., Osawa, T., Ogami, K., Ouyang, X., Sakaguchi, S., Koshiba, R., et al. (2008). A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proceedings of the National Academy of Sciences of the United States of America, 105(51), 20446–20451. doi:10.1073/pnas.0810372105.
Richer, M. J., Lavallee, D. J., Shanina, I., & Horwitz, M. S. (2009). Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PloS one, 4(1), e4127. doi:10.1371/journal.pone.0004127.
Abston, E. D., Coronado, M. J., Bucek, A., Bedja, D., Shin, J., Kim, J. B., et al. (2012). Th2 regulation of viral myocarditis in mice: different roles for TLR3 versus TRIF in progression to chronic disease. Clinical & developmental immunology, 2012, 129486. doi:10.1155/2012/129486.
Riad, A., Westermann, D., Zietsch, C., Savvatis, K., Becher, P. M., Bereswill, S., et al. (2011). TRIF is a critical survival factor in viral cardiomyopathy. Journal of Immunology, 186(4), 2561–2570. doi:10.4049/jimmunol.1002029.
Newman, K. C., & Riley, E. M. (2007). Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nature reviews Immunology, 7(4), 279–291. doi:10.1038/nri2057.
Wessely, R., Klingel, K., Knowlton, K. U., & Kandolf, R. (2001). Cardioselective infection with coxsackievirus B3 requires intact type I interferon signaling: implications for mortality and early viral replication. Circulation, 103(5), 756–761.
Deonarain, R., Cerullo, D., Fuse, K., Liu, P. P., & Fish, E. N. (2004). Protective role for interferon-beta in coxsackievirus B3 infection. Circulation, 110(23), 3540–3543. doi:10.1161/01.CIR.0000136824.73458.20.
Kuhl, U., Pauschinger, M., Schwimmbeck, P. L., Seeberg, B., Lober, C., Noutsias, M., et al. (2003). Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation, 107(22), 2793–2798. doi:10.1161/01.CIR.0000072766.67150.51.
Wang, Y. X., da Cunha, V., Vincelette, J., White, K., Velichko, S., Xu, Y., et al. (2007). Antiviral and myocyte protective effects of murine interferon-beta and -{alpha}2 in coxsackievirus B3-induced myocarditis and epicarditis in Balb/c mice. American journal of physiology Heart and circulatory physiology, 293(1), H69–76. doi:10.1152/ajpheart.00154.2007.
Kuhl, U., Lassner, D., von Schlippenbach, J., Poller, W., & Schultheiss, H. P. (2012). Interferon-beta improves survival in enterovirus-associated cardiomyopathy. Journal of the American College of Cardiology, 60(14), 1295–1296. doi:10.1016/j.jacc.2012.06.026.
Fensterl, V., & Sen, G. C. (2009). Interferons and viral infections. Biofactors, 35(1), 14–20. doi:10.1002/biof.6.
Yuan, J., Liu, Z., Lim, T., Zhang, H., He, J., Walker, E., et al. (2009). CXCL10 inhibits viral replication through recruitment of natural killer cells in coxsackievirus B3-induced myocarditis. Circulation research, 104(5), 628–638. doi:10.1161/CIRCRESAHA.108.192179.
Godeny, E. K., & Gauntt, C. J. (1987). Murine natural killer cells limit coxsackievirus B3 replication. Journal of Immunology, 139(3), 913–918.
Mackman, N. (2006). Role of tissue factor in hemostasis and thrombosis. Blood cells, molecules & diseases, 36(2), 104–107. doi:10.1016/j.bcmd.2005.12.008.
Drake, T. A., Morrissey, J. H., & Edgington, T. S. (1989). Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. The American journal of pathology, 134(5), 1087–1097.
Fleck, R. A., Rao, L. V., Rapaport, S. I., & Varki, N. (1990). Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thrombosis research, 59(2), 421–437.
Degen, J. L., Bugge, T. H., & Goguen, J. D. (2007). Fibrin and fibrinolysis in infection and host defense. Journal of thrombosis and haemostasis : JTH, 5(Suppl 1), 24–31. doi:10.1111/j.1538-7836.2007.02519.x.
Esmon, C. T., Xu, J., & Lupu, F. (2011). Innate immunity and coagulation. Journal of thrombosis and haemostasis : JTH, 9(Suppl 1), 182–188. doi:10.1111/j.1538-7836.2011.04323.x.
Flick, M. J., Du, X., Witte, D. P., Jirouskova, M., Soloviev, D. A., Busuttil, S. J., et al. (2004). Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. The Journal of clinical investigation, 113(11), 1596–1606. doi:10.1172/JCI20741.
Palumbo, J. S., Talmage, K. E., Massari, J. V., La Jeunesse, C. M., Flick, M. J., Kombrinck, K. W., et al. (2005). Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood, 105(1), 178–185. doi:10.1182/blood-2004-06-2272.
Key, N. S., Vercellotti, G. M., Winkelmann, J. C., Moldow, C. F., Goodman, J. L., Esmon, N. L., et al. (1990). Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proceedings of the National Academy of Sciences of the United States of America, 87(18), 7095–7099.
Geisbert, T. W., Young, H. A., Jahrling, P. B., Davis, K. J., Kagan, E., & Hensley, L. E. (2003). Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. The Journal of infectious diseases, 188(11), 1618–1629. doi:10.1086/379724.
Goeijenbier, M., van Wissen, M., van de Weg, C., Jong, E., Gerdes, V. E., Meijers, J. C., et al. (2012). Review: viral infections and mechanisms of thrombosis and bleeding. Journal of medical virology, 84(10), 1680–1696. doi:10.1002/jmv.23354.
Funderburg, N. T., Mayne, E., Sieg, S. F., Asaad, R., Jiang, W., Kalinowska, M., et al. (2010). Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood, 115(2), 161–167. doi:10.1182/blood-2009-03-210179.
Antoniak, S., Owens, A. P., 3rd, Baunacke, M., Williams, J. C., Lee, R. D., Weithauser, A., et al. (2013). PAR-1 contributes to the innate immune response during viral infection. The Journal of clinical investigation, 123(3), 1310–1322. doi:10.1172/JCI66125.
Shibamiya, A., Hersemeyer, K., Schmidt Woll, T., Sedding, D., Daniel, J. M., Bauer, S., et al. (2009). A key role for Toll-like receptor-3 in disrupting the hemostasis balance on endothelial cells. Blood, 113(3), 714–722. doi:10.1182/blood-2008-02-137901.
Zare, F., Magnusson, M., Mollers, L. N., Jin, T., Tarkowski, A., & Bokarewa, M. (2008). Single-stranded polyinosinic acid oligonucleotides trigger leukocyte production of proteins belonging to fibrinolytic and coagulation cascades. Journal of leukocyte biology, 84(3), 741–747. doi:10.1189/jlb.0506345.
Kato, J., Okamoto, T., Motoyama, H., Uchiyama, R., Kirchhofer, D., Van Rooijen, N., et al. (2013). Interferon-gamma-mediated tissue factor expression contributes to T-cell-mediated hepatitis through induction of hypercoagulation in mice. Hepatology, 57(1), 362–372. doi:10.1002/hep.26027.
Bhattacharyya, M., Karmohapatra, S. K., Bhattacharya, G., Bhattacharya, R., & Sinha, A. K. (2009). The role of leucocytes in the acetyl salicylic acid (aspirin) induced nitric oxide synthesis in the production of interferon-alpha, a potent inhibitor of platelet aggregation and a thrombolytic agent. Journal of thrombosis and thrombolysis, 28(2), 173–184. doi:10.1007/s11239-008-0283-1.
Antoniak, S., Boltzen, U., Eisenreich, A., Stellbaum, C., Poller, W., Schultheiss, H. P., et al. (2009). Regulation of cardiomyocyte full-length tissue factor expression and microparticle release under inflammatory conditions in vitro. Journal of thrombosis and haemostasis : JTH, 7(5), 871–878. doi:10.1111/j.1538-7836.2009.03323.x.
Tomioka, N., Kishimoto, C., Matsumori, A., & Kawai, C. (1985). Mural thrombi in mice with acute viral myocarditis. Japanese circulation journal, 49(12), 1277–1279.
Kojima, J., Miyazaki, S., Fujiwara, H., Kumada, T., & Kawai, C. (1988). Recurrent left ventricular mural thrombi in a patient with acute myocarditis. Heart and vessels, 4(2), 120–122.
Mazza, I. L., Jacobs, J. P., Aldousany, A., Chang, A. C., & Burke, R. P. (1998). Video-assisted cardioscopy for left ventricular thrombectomy in a child. The Annals of thoracic surgery, 66(1), 248–250.
Weikert, U., Rauch, U., Kuhl, U., Hohmann, C., Jaster, M., Pauschinger, M., et al. (2002). Increased thrombocyte activation in dilated cardiomyopathy: a risk factor for development of ventricular thrombosis despite anticoagulant therapy? Zeitschrift für Kardiologie, 91(5), 423–429.
Miyamoto, K., Yasuda, S., Noguchi, T., Tanimoto, T., Kakuchi, H., Morii, I., et al. (2005). Fulminant myocarditis causing severe left heart failure and massive thrombus formation following cardiac tamponade: a case report. Journal of cardiology, 46(1), 25–31.
Kuh, J. H., & Seo, Y. (2005). Transatrial resection of a left ventricular thrombus after acute myocarditis. Heart and vessels, 20(5), 230–232. doi:10.1007/s00380-004-0811-7.
Lin, K. Y., Kerur, B., Witmer, C. M., Beslow, L. A., Licht, D. J., Ichord, R. N., & Kaufman, B. D. (2013). Thrombotic events in critically ill children with myocarditis. Cardiology in the Young, 9, 1–8. doi:10.1017/S1047951113001145.
Gottdiener, J. S., Gay, J. A., VanVoorhees, L., DiBianco, R., & Fletcher, R. D. (1983). Frequency and embolic potential of left ventricular thrombus in dilated cardiomyopathy: assessment by 2-dimensional echocardiography. The American journal of cardiology, 52(10), 1281–1285.
Tomioka, N., Kishimoto, C., Matsumori, A., & Kawai, C. (1986). Mural thrombus in experimental viral myocarditis in mice: relation between thrombosis and congestive heart failure. Cardiovascular research, 20(9), 665–671.
Kishimoto, C., Ochiai, H., & Sasayama, S. (1992). Intracardiac thrombus in murine coxsackievirus B3 myocarditis. Heart and vessels, 7(2), 76–81.
Nakamura, Y., Nakamura, K., Fukushima-Kusano, K., Ohta, K., Matsubara, H., Hamuro, T., et al. (2003). Tissue factor expression in atrial endothelia associated with nonvalvular atrial fibrillation: possible involvement in intracardiac thrombogenesis. Thrombosis research, 111(3), 137–142.
Szotowski, B., Goldin-Lang, P., Antoniak, S., Bogdanov, V. Y., Pathirana, D., Pauschinger, M., et al. (2005). Alterations in myocardial tissue factor expression and cellular localization in dilated cardiomyopathy. Journal of the American College of Cardiology, 45(7), 1081–1089. doi:10.1016/j.jacc.2004.12.061.
Weikert, U., Kuhl, U., Schultheiss, H. P., & Rauch, U. (2002). Platelet activation is increased in patients with cardiomyopathy: myocardial inflammation and platelet reactivity. Platelets, 13(8), 487–491. doi:10.1080/0953710021000057857.
Schnitt, S. J., Stillman, I. E., Owings, D. V., Kishimoto, C., Dvorak, H. F., & Abelmann, W. H. (1993). Myocardial fibrin deposition in experimental viral myocarditis that progresses to dilated cardiomyopathy. Circulation research, 72(4), 914–920.
Frizelle, S., Schwarz, J., Huber, S. A., & Leslie, K. (1992). Evaluation of the effects of low molecular weight heparin on inflammation and collagen deposition in chronic coxsackievirus B3-induced myocarditis in A/J mice. The American journal of pathology, 141(1), 203–209.
Szotowski, B., Antoniak, S., Poller, W., Schultheiss, H. P., & Rauch, U. (2005). Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circulation research, 96(12), 1233–1239. doi:10.1161/01.RES.0000171805.24799.fa.
Coughlin, S. R. (2000). Thrombin signalling and protease-activated receptors. Nature, 407(6801), 258–264. doi:10.1038/35025229.
Antoniak, S., Pawlinski, R., & Mackman, N. (2011). Protease-activated receptors and myocardial infarction. IUBMB life, 63(6), 383–389. doi:10.1002/iub.441.
Soh, U. J., Dores, M. R., Chen, B., & Trejo, J. (2010). Signal transduction by protease-activated receptors. British journal of pharmacology, 160(2), 191–203. doi:10.1111/j.1476-5381.2010.00705.x.
Kahn, M. L., Zheng, Y. W., Huang, W., Bigornia, V., Zeng, D., Moff, S., et al. (1998). A dual thrombin receptor system for platelet activation. Nature, 394(6694), 690–694. doi:10.1038/29325.
Steinhoff, M., Buddenkotte, J., Shpacovitch, V., Rattenholl, A., Moormann, C., Vergnolle, N., et al. (2005). Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocrine reviews, 26(1), 1–43. doi:10.1210/er.2003-0025.
Shpacovitch, V., Feld, M., Bunnett, N. W., & Steinhoff, M. (2007). Protease-activated receptors: novel PARtners in innate immunity. Trends in immunology, 28(12), 541–550. doi:10.1016/j.it.2007.09.001.
Moretti, S., Bellocchio, S., Bonifazi, P., Bozza, S., Zelante, T., Bistoni, F., et al. (2008). The contribution of PARs to inflammation and immunity to fungi. Mucosal immunology, 1(2), 156–168. doi:10.1038/mi.2007.13.
Day, J. R., Landis, R. C., & Taylor, K. M. (2006). Aprotinin and the protease-activated receptor 1 thrombin receptor: antithrombosis, inflammation, and stroke reduction. Seminars in cardiothoracic and vascular anesthesia, 10(2), 132–142. doi:10.1177/1089253206288997.
Pan, H. Y., Yamada, H., Chida, J., Wang, S., Yano, M., Yao, M., et al. (2011). Up-regulation of ectopic trypsins in the myocardium by influenza A virus infection triggers acute myocarditis. Cardiovascular research, 89(3), 595–603. doi:10.1093/cvr/cvq358.
Pan, H. Y., Yano, M., & Kido, H. (2011). Effects of inhibitors of Toll-like receptors, protease-activated receptor-2 signalings and trypsin on influenza A virus replication and upregulation of cellular factors in cardiomyocytes. The journal of medical investigation : JMI, 58(1–2), 19–28.
Seitz, C., Isken, B., Heynisch, B., Rettkowski, M., Frensing, T., & Reichl, U. (2012). Trypsin promotes efficient influenza vaccine production in MDCK cells by interfering with the antiviral host response. Applied microbiology and biotechnology, 93(2), 601–611. doi:10.1007/s00253-011-3569-8.
Sun, X., Tse, L. V., Ferguson, A. D., & Whittaker, G. R. (2010). Modifications to the hemagglutinin cleavage site control the virulence of a neurotropic H1N1 influenza virus. Journal of virology, 84(17), 8683–8690. doi:10.1128/JVI.00797-10.
Gomides, L. F., Duarte, I. D., Ferreira, R. G., Perez, A. C., Francischi, J. N., & Klein, A. (2012). Proteinase-activated receptor-4 plays a major role in the recruitment of neutrophils induced by trypsin or carrageenan during pleurisy in mice. Pharmacology, 89(5–6), 275–282. doi:10.1159/000337378.
Sambrano, G. R., Huang, W., Faruqi, T., Mahrus, S., Craik, C., & Coughlin, S. R. (2000). Cathepsin G activates protease-activated receptor-4 in human platelets. The Journal of biological chemistry, 275(10), 6819–6823.
Fairweather, D., Frisancho-Kiss, S., Yusung, S. A., Barrett, M. A., Davis, S. E., Steele, R. A., et al. (2005). IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. Journal of Immunology, 174(1), 261–269.
Grabie, N., Hsieh, D. T., Buono, C., Westrich, J. R., Allen, J. A., Pang, H., et al. (2003). Neutrophils sustain pathogenic CD8+ T cell responses in the heart. The American journal of pathology, 163(6), 2413–2420. doi:10.1016/S0002-9440(10)63596-1.
Dulon, S., Cande, C., Bunnett, N. W., Hollenberg, M. D., Chignard, M., & Pidard, D. (2003). Proteinase-activated receptor-2 and human lung epithelial cells: disarming by neutrophil serine proteinases. American journal of respiratory cell and molecular biology, 28(3), 339–346. doi:10.1165/rcmb.4908.
Suzuki, T., Yamashita, C., Zemans, R. L., Briones, N., Van Linden, A., & Downey, G. P. (2009). Leukocyte elastase induces lung epithelial apoptosis via a PAR-1-, NF-kappaB-, and p53-dependent pathway. American journal of respiratory cell and molecular biology, 41(6), 742–755. doi:10.1165/rcmb.2008-0157OC.
Lee, J. K., Zaidi, S. H., Liu, P., Dawood, F., Cheah, A. Y., Wen, W. H., et al. (1998). A serine elastase inhibitor reduces inflammation and fibrosis and preserves cardiac function after experimentally-induced murine myocarditis. Nature medicine, 4(12), 1383–1391. doi:10.1038/3973.
Marchant, D., & McManus, B. M. (2009). Matrix metalloproteinases in the pathogenesis of viral heart disease. Trends in cardiovascular medicine, 19(1), 21–26. doi:10.1016/j.tcm.2009.04.001.
Jaffre, F., Friedman, A. E., Hu, Z., Mackman, N., & Blaxall, B. C. (2012). Beta-adrenergic receptor stimulation transactivates protease-activated receptor 1 via matrix metalloproteinase 13 in cardiac cells. Circulation, 125(24), 2993–3003. doi:10.1161/CIRCULATIONAHA.111.066787.
Yang, E., Boire, A., Agarwal, A., Nguyen, N., O’Callaghan, K., Tu, P., et al. (2009). Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer research, 69(15), 6223–6231. doi:10.1158/0008-5472.CAN-09-0187.
Sharma, R., Prasad, V., McCarthy, E. T., Savin, V. J., Dileepan, K. N., Stechschulte, D. J., et al. (2007). Chymase increases glomerular albumin permeability via protease-activated receptor-2. Molecular and cellular biochemistry, 297(1–2), 161–169. doi:10.1007/s11010-006-9342-0.
Molino, M., Barnathan, E. S., Numerof, R., Clark, J., Dreyer, M., Cumashi, A., et al. (1997). Interactions of mast cell tryptase with thrombin receptors and PAR-2. The Journal of biological chemistry, 272(7), 4043–4049.
Fairweather, D., Frisancho-Kiss, S., Gatewood, S., Njoku, D., Steele, R., Barrett, M., et al. (2004). Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity, 37(2), 131–145.
Higuchi, H., Hara, M., Yamamoto, K., Miyamoto, T., Kinoshita, M., Yamada, T., et al. (2008). Mast cells play a critical role in the pathogenesis of viral myocarditis. Circulation, 118(4), 363–372. doi:10.1161/CIRCULATIONAHA.107.741595.
Gan, X., Liu, D., Huang, P., Gao, W., Chen, X., & Hei, Z. (2012). Mast-cell-releasing tryptase triggers acute lung injury induced by small intestinal ischemia-reperfusion by activating PAR-2 in rats. Inflammation, 35(3), 1144–1153. doi:10.1007/s10753-011-9422-5.
He, S., Aslam, A., Gaca, M. D., He, Y., Buckley, M. G., Hollenberg, M. D., et al. (2004). Inhibitors of tryptase as mast cell-stabilizing agents in the human airways: effects of tryptase and other agonists of proteinase-activated receptor 2 on histamine release. The Journal of pharmacology and experimental therapeutics, 309(1), 119–126. doi:10.1124/jpet.103.061291.
Bazan, J. F., & Fletterick, R. J. (1988). Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications. Proceedings of the National Academy of Sciences of the United States of America, 85(21), 7872–7876.
Thibaut, H. J., De Palma, A. M., & Neyts, J. (2012). Combating enterovirus replication: state-of-the-art on antiviral research. Biochemical pharmacology, 83(2), 185–192. doi:10.1016/j.bcp.2011.08.016.
Liang, G., Barker, T., Xie, Z., Charles, N., Rivera, J., & Druey, K. M. (2012). Naive T cells sense the cysteine protease allergen papain through protease-activated receptor 2 and propel TH2 immunity. The Journal of allergy and clinical immunology, 129(5), 1377–1386 e1313. doi:10.1016/j.jaci.2012.02.035.
Xing, Y., Chen, J., Tu, J., Zhang, B., Chen, X., Shi, H., et al. (2013). The papain-like protease of porcine epidemic diarrhea virus negatively regulates type I interferon pathway by acting as a viral deubiquitinase. The Journal of general virology, 94(Pt 7), 1554–1567. doi:10.1099/vir.0.051169-0.
Schechter, N. M., Brass, L. F., Lavker, R. M., & Jensen, P. J. (1998). Reaction of mast cell proteases tryptase and chymase with protease activated receptors (PARs) on keratinocytes and fibroblasts. Journal of cellular physiology, 176(2), 365–373. doi:10.1002/(SICI)1097-4652(199808)176:2<365::AID-JCP15>3.0.CO;2-2.
Sutherland, M. R., Friedman, H. M., & Pryzdial, E. L. (2007). Thrombin enhances herpes simplex virus infection of cells involving protease-activated receptor 1. Journal of thrombosis and haemostasis : JTH, 5(5), 1055–1061. doi:10.1111/j.1538-7836.2007.02441.x.
Naldini, A., & Carney, D. H. (1996). Thrombin modulation of natural killer activity in human peripheral lymphocytes. Cellular immunology, 172(1), 35–42. doi:10.1006/cimm.1996.0212.
Sutherland, M. R., Ruf, W., & Pryzdial, E. L. (2012). Tissue factor and glycoprotein C on herpes simplex virus type 1 are protease-activated receptor 2 cofactors that enhance infection. Blood, 119(15), 3638–3645. doi:10.1182/blood-2011-08-376814.
Weithaeuser, A., Bobbert, P., Antoniak, S., Boehm, A., Rauch, B.H., Klingel, K., Savvatis, K., Kroemer, H.K., Tschope, C., Stroux, A., Zeichhardt, H., Poller, W., Mackman, N., Schultheiss, H.P., Rauch, U. (2013). Protease-activated receptor 2 regulates the Innate Immune Response to Viral Infection in a CVB3-induced Myocarditis. Journal of the American College of Cardiology, 62(19), 1737–1745. doi:10.1016/j.jacc.2013.05.076.
Nhu, Q. M., Shirey, K., Teijaro, J. R., Farber, D. L., Netzel-Arnett, S., Antalis, T. M., et al. (2010). Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal immunology, 3(1), 29–39. doi:10.1038/mi.2009.120.
Rallabhandi, P., Nhu, Q. M., Toshchakov, V. Y., Piao, W., Medvedev, A. E., Hollenberg, M. D., et al. (2008). Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. The Journal of biological chemistry, 283(36), 24314–24325. doi:10.1074/jbc.M804800200.
Williams, J. C., Lee, R. D., Doerschuk, C. M., & Mackman, N. (2011). Effect of PAR-2 Deficiency in Mice on KC Expression after Intratracheal LPS Administration. Journal of signal transduction, 2011, 415195. doi:10.1155/2011/415195.
Eriksson, U., Ricci, R., Hunziker, L., Kurrer, M. O., Oudit, G. Y., Watts, T. H., et al. (2003). Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nature medicine, 9(12), 1484–1490. doi:10.1038/nm960.
Fuse, K., Chan, G., Liu, Y., Gudgeon, P., Husain, M., Chen, M., et al. (2005). Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation, 112(15), 2276–2285. doi:10.1161/CIRCULATIONAHA.105.536433.
Frisancho-Kiss, S., Davis, S. E., Nyland, J. F., Frisancho, J. A., Cihakova, D., Barrett, M. A., et al. (2007). Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. Journal of Immunology, 178(11), 6710–6714.
Roberts, B. J., Dragon, J. A., Moussawi, M., & Huber, S. A. (2012). Sex-specific signaling through Toll-Like receptors 2 and 4 contributes to survival outcome of coxsackievirus B3 infection in C57Bl/6 mice. Biology of sex differences, 3(1), 25. doi:10.1186/2042-6410-3-25.
Cimmino, G., & Golino, P. (2013). Platelet biology and receptor pathways. Journal of cardiovascular translational research, 6(3), 299–309. doi:10.1007/s12265-012-9445-9.
Smyth, S. S., McEver, R. P., Weyrich, A. S., Morrell, C. N., Hoffman, M. R., Arepally, G. M., et al. (2009). Platelet functions beyond hemostasis. Journal of thrombosis and haemostasis : JTH, 7(11), 1759–1766. doi:10.1111/j.1538-7836.2009.03586.x.
Li, C., Li, J., Li, Y., Lang, S., Yougbare, I., Zhu, G., et al. (2012). Crosstalk between platelets and the immune system: old systems with new discoveries. Advances in hematology, 2012, 384685. doi:10.1155/2012/384685.
Sabri, A., Guo, J., Elouardighi, H., Darrow, A. L., Andrade-Gordon, P., & Steinberg, S. F. (2003). Mechanisms of protease-activated receptor-4 actions in cardiomyocytes. Role of Src tyrosine kinase. The Journal of biological chemistry, 278(13), 11714–11720. doi:10.1074/jbc.M213091200.
Meune, C., Spaulding, C., Mahe, I., Lebon, P., & Bergmann, J. F. (2003). Risks versus benefits of NSAIDs including aspirin in myocarditis: a review of the evidence from animal studies. Drug safety : an international journal of medical toxicology and drug experience, 26(13), 975–981.
Eyers, S., Weatherall, M., Shirtcliffe, P., Perrin, K., & Beasley, R. (2010). The effect on mortality of antipyretics in the treatment of influenza infection: systematic review and meta-analysis. Journal of the Royal Society of Medicine, 103(10), 403–411. doi:10.1258/jrsm.2010.090441.
Shimazu, T. (2009). A/H1N1 flu. Aspirin in the 1918 pandemic. BMJ, 338, b2398.
Starko, K. M. (2009). Salicylates and pandemic influenza mortality, 1918–1919 pharmacology, pathology, and historic evidence. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 49(9), 1405–1410. doi:10.1086/606060.
Weitz, J. I., & Gross, P. L. (2012). New oral anticoagulants: which one should my patient use? Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program, 2012, 536–540. doi:10.1182/asheducation-2012.1.536.
Connolly, S., Ezekowitz, M., Brueckmann, M., Clemens, A., Yusuf, S., Wallentin, L. (2013). eLetter Re: Antoniak et al. PAR-1 contributes to the inate immune response during viral infection. The Journal of clinical investigation http://www.jci.org/eletters/view/66125#sec1. Accessed 1 May 2013.
Malz, R., Becher, M., Westermann, D., Schultheiss, H.P., Rauch, U. (2012). Inhibition of coagulation factor Xa improves left ventricular function during CVB3-induced myocarditis [abstract]. Paper presented at the 78. Jahrestagung der DGK, 2012
Mason, J. W. (2013). Basic research on myocarditis: superb but unrequited. Journal of the American College of Cardiology. doi:10.1016/j.jacc.2013.06.030.
Lohman, R. J., Cotterell, A. J., Barry, G. D., Liu, L., Suen, J. Y., Vesey, D. A., et al. (2012). An antagonist of human protease activated receptor-2 attenuates PAR2 signaling, macrophage activation, mast cell degranulation, and collagen-induced arthritis in rats. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 26(7), 2877–2887. doi:10.1096/fj.11-201004.
Sevigny, L. M., Zhang, P., Bohm, A., Lazarides, K., Perides, G., Covic, L., et al. (2011). Interdicting protease-activated receptor-2-driven inflammation with cell-penetrating pepducins. Proceedings of the National Academy of Sciences of the United States of America, 108(20), 8491–8496. doi:10.1073/pnas.1017091108.
Acknowledgments
We thank Dr. Julie C. Williams for her careful and critical review of this manuscript. This work was supported by grants from the Myocarditis Foundation (to S. Antoniak) and the National Institutes of Health (to N. Mackman, HL119523).
Conflict of Interests
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Daniel P. Judge oversaw the review of this article
Rights and permissions
About this article
Cite this article
Antoniak, S., Mackman, N. Coagulation, Protease-Activated Receptors, and Viral Myocarditis. J. of Cardiovasc. Trans. Res. 7, 203–211 (2014). https://doi.org/10.1007/s12265-013-9515-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-013-9515-7