Abstract
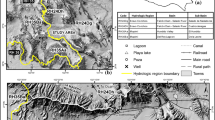
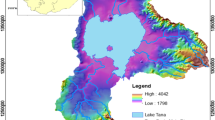
In this work, we present results of the hydrogeochemical and isotopic studies on groundwater samples from the El Ma El Abiod Sandstone aquifer, in Tébessa, Algeria. Chemical and environmental isotope data are presented and discussed in order to identify the geochemical processes and their relation with groundwater quality as well as to get an insight into the hydrochemical evaluation, in space and time, of groundwater and of the origin of dissolved species. A combined hydrogeologic and isotopic investigation have been carried out using chemical and isotopic data to deduce a hydrochemical evaluation of the aquifer system based on the ionic constituents, water types, hydrochemical facies, and factors controlling groundwater quality. All of the investigated groundwaters are categorized into two chemical types: low mineralized water type and relatively high mineralized water type. Interpretation of chemical data, based on thermodynamic calculations and geochemical reaction models of selected water groups constructed using PHREEQC, suggest that the chemical evolution of groundwater is primarily controlled by water–rock interactions, involving (1) acidic weathering of aluminosilicates, (2) dissolution of secondary carbonate minerals, and (3) cation exchange of Na+ for Ca2+. However, the original composition of groundwater may have been modified by further secondary processes such as mixing of chemically different water masses. The combined use of SI and mass-balance modeling has shown to be a useful approach in interpreting groundwater hydrochemistry in an area where large uncertainties exist in the understanding of the groundwater flow system. Interpretation of 18O and 2H, suggest that the recharge of the investigated groundwaters may result from different mechanisms.
ملخص
يقدم هذا العمل نتائجا هيدروجيوكيمائية و أخرى لنظائر عناصر كيمائية تخص الخزان الرملي لمنطقة الماء الأبيض بولاية تبسة الجزائرية. كل النتائج قدمت و نوقشت لأجل التعريف باللأليات الكيمائية و علاقتها بنوعية المياه الجوفية إلى جانب تطورها الميداني و الزمني من خلال أصل العناصر الكيمائية المذابة.
أضهرت عينات المياه المدروسة نوعان مختلفان من المياه‚ قسم قليل الأملاح و ثان على قدر كبير من الأملاح المذابة.
أوضحت الشروحات الترموديناميكية‚ عن طريق حساب التفاعلات باستخدام برنامج PHREEQC و نموذج هيدروكيمائي‚ أن تركيبة المياه تخضع لنوعية الخزان و تحليل بعض الأنواع المعدنية إلى جانب تبادل العناصر بين الصخرة الخازنة و المياه المختزنة. النظائر المستعملة لم تقدم شرحا دقيقا بل أكدت أن أصل مياه الخزان يعود إلى ميكانزمات عديدة.




Similar content being viewed by others
References
Appelo CAJ, Williemsen A (1987) Geochemical calculations and observations on salt water intrusions, a combined geochemical mixing cell model. J Hydro I94:313–330
Bender MM (1971) Variations in the 13C/t2C ratios of plants in reaction to the pathway of photosynthetic carbon dioxide fixation. Photochemistry 10:1239–1244
Craig H (1961) Isotopic variations in meteoric water. Science 133:1702–1703
Coleman et al (1982) Réduction de l'eau avec du zinc pour l'analyse des isotopes de l'hydrogène. Anal Chem 54:993–995
Debye, Hückel (1923) The theory of electrolytes. I. Lowering of freezing point and related phenomena. Phys Z 24:185–206
Edmunds WM, Shand P, Guendouz AH, Moula A, Mamou A, Zouari K (1997) Recharge characteristics and groundwater quality of the grand Erg oriental basin. Tech. Rep. Wd/97/46R, Vienna
Edmunds WM, Guendouz A, Mamou A, Moulla AS, Shand P, Zouari K (2003) Groundwater evolution in the continental intercalaire aquifer of southern Algeria and Tunisia: trace element and isotopic indicators. Appl Geochem 18(6):805–822
Epstein S, Mayeda TK (1953) Variations of the JSO/160 ratios in natural waters. Geochim Cosmochim Acta 4:213–224
Fritz B (1975) Etude thermodynamique et simulation des réactions entre minéraux et solutions$ Application à la géochimie des altérations et des eaux continentales. Mém Sci Géol Univ Strasbourg 41:153
Morel F, Morgan JJ (1972) A numerical method for computing equilibria in aqueous chemical systems. Environ Sci Teehnol 6:58–67
Nordstrom DK, Ball JW (1984) Chemical models, computer programs and metal complexation in natural water. In: Kramer CJM, Duniker J (eds) International symposium on trace metal complexation in natural water. Nijhoff/Junk, The Hague, pp 149–169
Plummer LN, Jones BF, Trusedell AH (1976) WATEQ—a Fortran IV version of WATEQC computer program for calculating chemical equilibrium of natural waters, vol. 76. USGS Water Resources, Washington, pp 13–61 (Revised 1978, 1984)
Plummer LN, Parkhurst DL, Thorstensen DC (1983) Development of reaction models for groundwater systems. Geochim Cosmochim Acta 47:665–685
Plummer, L.N., Prestemon, E.C. and Parkhurst, D.L. (1991) An interactive code (NETPATH) for modeling NETgeochemical reactions along a flow PATH. USGS Water Resources Investigations Report 91-4078.
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2): a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. USGS Water Resources Investigations Report 99-4259
Paschke SS, van der Heide P (1996) Overview of chemical modeling in groundwater and listing of available geochemical models. Report GWMI-96-01, International Groundwater Modeling Center, Colorado School of Mines, Golden, Co.
Güler C, Thyne GD (2004) Hydrologic and geologic factors controlling surface and groundwater chemistry in Indian Wells-Owens Valley area, southeastern California, USA. J Hydrol 285:177–198
Rouabhia A (2001) Vulnérabilité à la pollution chimique d’un système aquifère en région semi-aride d’Algérie. Cas de la plaine d’El Ma El Abiod. Mém. de Magister. Université de Annaba, p. 120
Rouabhia A (2006) Vulnérabilité et risque de pollution des eaux souterraines de la nappe des sables miocènes de la plaine d'El Ma El Abiod (Algérie). Doctorat Thesis, University of Annaba Algeria. p 210
Rouabhia A, Fehdi Ch, Baali F, Djabri L, Rouabhi R (2008) Impact of human activities on quality and geochemistry of groundwater in the Merdja area, Tebessa, Algeria. Environ Geol. ISSN 0943-0105 (Print) 1432-0495 (Online). doi:10.1007/s00254-008-1225-0
Truesdell AH, Jones BF (1974) WATEQ, a computer program for calculating chemical equilibria in natural waters. J Res US Geol Surv 2:233–274
Zhu C, Anderson G (2002) Environmental applications of geochemical modeling. Cambridge University Press, Cambridge
Acknowledgements
The authors thank the anonymous reviewers for their constructive comments. This work has been realized through the framework of two CNPRU projects G02920070001 (2008) and G02920060020 (2007). We would like to thank Profs. Djabri L., HANI A. and Kherici N. (Algeria) and Ouddane B. (France).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rouabhia, A., Baali, F., Fehdi, C. et al. Hydrogeochemistry of groundwaters in a semi-arid region. El Ma El Abiod aquifer, Eastern Algeria. Arab J Geosci 4, 973–982 (2011). https://doi.org/10.1007/s12517-010-0169-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-010-0169-3