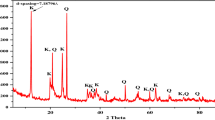
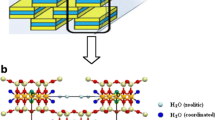
Abstract
Clays, particularly kaolinite, are promising adsorbents for the treatment of textile effluents, but there is a need of better understanding the mechanisms of adsorption, especially in the case of anionic dyes. Thus, the removal of RR120 anionic dye was investigated using Tunisian raw clay (TBK) composed of kaolinite and illite, and a standard kaolinite (KGa-2), and conducting batch experiments by varying different parameters (contact time, ionic strength, concentration, temperature). We investigated the clays’ surface charges by electrophoretic mobility measures and the dye-clay interactions during adsorption, by the streaming-induced potentials (SIP). The results showed that KGa-2 has higher adsorption capacity for RR120 dye than TBK clay, moreover enhanced by increasing the ionic strength and/or lowering the pH of the aqueous. The SIP results showed an increase of negative charges for both clays, reflecting the adsorption of the anionic dye on the positive charges of the amphoteric surfaces of the clays. The SIP magnitudes indicated a higher adsorption rate for KGa-2 in accordance with the kinetic study. The Sips model that described the best adsorption isotherms indicates lateral interactions of the dye molecules, stronger in the case of KGa-2 than TBK. Also, the dye molecules form a thinner layer on KGa-2 surfaces. In addition, the dye molecule’s structure was not altered, as verified by mass spectrometry. The adsorption process was feasible and spontaneous and favored at ambient temperature. Thus, kaolinite-rich clays are effective in the removal of anionic dyes in aqueous solution and potential good adsorbents in wastewater treatment.









Similar content being viewed by others
References
Abd El-Rahim WM, Moawad H, Khalafallah MA (2003) Enhancing the growth of fungal promising strains for rapid dye removal. Fresenius Environ Bull (FEB) 12:764–770
Abidi N, Duplay J, Ayari F, Gangloff S, Trabelsi-Ayadi M (2014) Adsorption of anionic dye on natural and organophilic clays: effect of textile dyeing additives. Desalin Water Treat 54(6):1–16
Abidi N, Errais E, Duplay J, Berez A, Jrad J, Schäfer G, Ghazi M, Semhi K, Trabelsi-Ayadi M (2015) Treatment of dye-containing effluent by natural clay. J Clean Prod 86:432–440
Adamson AW, Gast AP (1997) Physical chemistry of surfaces, sixth edn. Wiley, New York
AFNOR. NF X 31-130 (1999) Soil quality. Chemical methods. Determination of cation exchange capacity (CEC) and cation extraction. AFNOR, Paris
Al G, Özdemir U, Aksoy Ö (2013) Cytotoxic effects of Reactive Blue 33 on Allium cepa determined using Taguchi’s L8 orthogonal array. Ecotoxicol Environ 98:36–40
Allen SJ, Koumanova B (2005) Decolourisation of water/wastewater using adsorption. J Univ ChemTechnol Metall 40(3):175–192
Alshameri A, Abood AR, Yan C, Muhammad AM (2014) Characteristics, modification and environmental application of Yemen’s natural bentonite. Arab J Geosci 7:841–853
Attia AA, Girgis BS, Khedr SA (2003) Capacity of activated carbon derived from pistachio shells by H3PO4 in the removal of dyes and phenolics. J Chem Technol Biotechnol 78:611–619
Bazin I, Andreotti N, Ibn HadjHassine A, DE Waard M, Sabatier JM, Gonzalez C (2012) The assessment of estrogenic and anti-estrogenic activity of 23 commercial textile dyes industry. Ecotoxicol Environ Saf 85:131–113
Bhatacharyya KG, Sharma A (2004) Azardirachta indica leaf powder as an effective biosorbent for dyes: a case study with aqueous Congo red solution. J Environ Manag 71:217–229
Bouberka Z, Kacha S, Kameche M, Elmaleh S, Derriche Z (2005) Sorption study of an acid dye from aqueous solutions using modified clays. J Hazard Mater B119:117–124
Bousher A, Shen X, Edyvean RGJ (1997) Removal of coloured organic matter by adsorption onto low-cost materials. Water Res 31:2084–2092
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multi-molecular layers. J Am Chem Soc 60:309–319
Campos Ventura-Camargo B, PasqualiPariseMaltempi P, Aparecida Marin-Morales M (2011) The use of the cytogenetic to identify mechanisms of action of an azo dye in Allium cepa meristematic cells. J Environ Anal Toxicol 1(3):1–3
Cao F, Wang Y, Ping Q, Liao Z (2011) Zn–Al–NO3-layered double hydroxides with intercalated diclofenac for delivery. Int J Pharm 404(1–2):250–256
Chaari I, Moussi B, Jamoussi F (2015) Interactions of the dye, C.I. direct orange 34 with natural clay. J Alloys Compd 647:720–727
Chiou MS, Chuang GS (2006) Competitive adsorption of dyemetanil yellow and RB15 in acid solutions on chemically cross-linked chitosan beads. Chemosphere 62:731–740
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
De Boer JH (1953) The thermodynamic character of adsorption. Oxford University Press, London
De Oliveira T, Guégan R (2016) Coupled organoclay/micelle action for the adsorption of diclofenac. Environ Sci Technol 50(18):10209–10215
Elovich S J (1959) Proceedings of the second international congress on surface activity. 11. In: Schulman JH (ed). Academic, New York, p 253
Errais E, Duplay J, Darragi F, M’Rabet I, Aubert A, Huber F, Morvan G (2011) Efficient anionic dye adsorption on natural untreated clay: kinetic study and thermodynamic parameters. Desalination 275:74–81
Errais E, Duplay J, Elhabiri M, Khodja M, Ocampo R, Baltenweck-Guyot R, Darragi F (2012) Anionic RR120 dye adsorption onto raw clay: surface properties and adsorption mechanism. Colloids Surf A: Physicochem Eng Asp 403:69–78
Ferrandon O, Bouabane H, Mazet M (1995) Tests for the validity of different models used for the adsorption of solutes on activated carbon. Rev Sci de L’eau 8:183–200
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. J Chem Eng 156:2–10
Fowler RH, Guggenheim EA (1965) Statistical thermodynamics, theory of the properties of matter in equilibrium. Cambridge Press, Cambridge
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Gundogan R, Acemioglu B, Alma MH (2004) Copper (II) adsorption from aqueous solution by herbaceous peat. J Colloid Interface Sci 269:303–309
Gupta SS, Bhattacharyya KG (2011) Kinetics of adsorption of metal ions on inorganic materials: a review. Adv Colloid Interf Sci 162:39–58
Gürses A, Dogar C, Yalcin M, Acikyildiz M, Bayrak R, Karaca S (2006) The adsorption kinetics of the cationic dye, methylene blue, onto clay. J Hazard Mater 13:217–228
Hill TL (1946) Localized and mobile adsorption and absorption. J Chem Phys 14:441–452
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. J Chem Eng 70:115–124
Jacobasch HJ, Bauböck G, Schurz J (1985) Problems and results of zeta-potential measurements on fibers. Colloid Polym Sci 3:263
Jada A, Erlenmeyer S (2012) Zeta potential of calcium carbonate precipitated in the presence of sodium polyacrylates. J Colloid Sci Biotechnol 1:129–136
Jada A, Debih H, Khodja M (2006) Montmorillonite surface properties modifications byasphaltenes adsorption. J Pet Sci Eng 52:305–316
Jossens L, Prausnitz JM, Fritz W, Schlünder EU, Myers AL (1978) Thermodynamics of multi-solute adsorption from dilute aqueous solutions. Chem Eng Sci 33:1097–1106
Khalfa L, Cervera ML, Bagane M, Souissi-Najar S (2016) Modeling of equilibrium isotherms and kinetic studies for Cr (VI) adsorption into natural and acid-activated clays. Arab J Geosci 9. doi:https://doi.org/10.1007/s12517015-2104-0
Khan AI, Lei L, Norquist AJ, O’Hare D (2001) Intercalation and controlled release of pharmaceutically active compounds from a layered double hydroxide. Chem Commun:2342–2343. doi:https://doi.org/10.1039/B106465G
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar Band 24:1–39
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Leroy P, Revil A (2004) A triple-layer model of the surface electrochemical properties of clay minerals. J Colloid and Interface Sci 270:371–380
Li Y, Shi JQ, Qu RJ, Feng MB, Liu F, Wang M, Wang ZY (2012) Toxicity assessment on three direct dyes (D-BLL, D-GLN, D-3RNL) using oxidative stress bioassay and quantum parameter calculation. Ecotoxicol Environ Saf 86:132–140
McKay G, Ho YS, Ng JCY (1999) Biosorption of copper from wastewaters: a review. Sep Purif Methods 28:87–125
Murat A (2014) Enhancement of the anionic dye adsorption capacity of clinoptiloliteby Fe3+-grafting. J Hazard Mater 267:1–8
Newcombe G, Drikas M (1997) Adsorption of NOM activated carbon: electrostatic and non-electrostatic effects. Carbon 35:1239–1250
Rahman A, Urabe T, Kishimoto N (2013) Color removal of reactive procion dyes by clay adsorbents. Procedia Environ Sci 17:270–278
Ridaoui H, Jada A, Vidal L, Donnet JB (2006) Effect of cationic surfactant and block copolymer on carbon black particle surface charge and size. Colloids Surf A Physicochem Eng Asp 278:149–159
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255
Saadi R, Saadi Z, Fazaeli R, ElmiFard N (2015) Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J Chem Eng 32(5):787–799
Salam MA, Mokhtar M, Basahel SN, Al-Thabaiti SA, Obaid AY (2010) Removal of chlorophenol from aqueous solutions by multi-walled carbonnanotubes: kinetic and thermodynamic studies. J Alloys Compd 500:87–92
Sips R (1948) Combined form of Langmuir and Freundlich equations. J Chem Phys 16:490–495
Sparado JT, Gold MH, Renganathan V (1992) Degradation of azo dyes by lignin-degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol 58:2397–2401
Ur Rehman MS, Munir M, Ashfaq M, Rashid N, Nazar MF, Danish M, Han JI (2013) Adsorption of brilliant green dye from aqueous solution onto red clay. J Chem Eng 228:54–62
Vajnhandl S, VolmajerValh J (2014) The status of water reuse in European textile sector. J Environ Manag 141:29–35
VolmajerValh J, Majcen Le Marechal A (2009) Decolouration of textile wastewaters, dyes and pigments new research. Nova Science Publisher, New York
Weber E J, Sturrock P E, Camp S R (1990) Reactive dyes in the aquatic environment: a case study of reactive blue 19, research brief. National Service Center for Environmental Publications (NSCEP), United States Environmental Protection Agency, EPA/600/M-90/009, 1990
Xue Y, Hou H, Zhu S (2009) Adsorption removal of reactive dyes from aqueous solution by modified basic oxygen furnace slag: isotherm and kinetic study. J Chem Eng 147:272–279
Yariv S, Cross H (2002) Organo-clay complexes and interactions, vol 39. Marcel Dekker, New York, p 688
Zaharia C and Suteu D (2012) Textile organic dyes—characteristics, polluting effects and separation/elimination procedures from industrial effluents—a critical overview, organic pollutants ten years after the Stockholm Convention—environmental and analytical update, Dr. Tomasz Puzyn (Ed.), ISBN:978–953–307-917-2, InTech, Available from: http://www.intechopen.com/books/organic-pollutants-ten-yearsafter-the-stockholm-convention-environmental-and-analytical-update/textile-organic- dyes-characteristicspolluting-effects-and-separation-elimination-procedures-from-in
Acknowledgments
This work was done in the frame of two projects: the ERANETMET SETPROpER project (2016–2019) with the support of the funding agencies of France (National Research Agency, ANR) and Tunisia (Ministry of Higher Education Scientific Research, and TIC TUNISIA); and the French-Tunisian PHC UTIQUE project (12G21002), with the support from the French Ministries of Foreign Affairs (MAE) and Education and Research (MESR), and the Tunisian Minister of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abidi, N., Duplay, J., Jada, A. et al. Toward the understanding of the treatment of textile industries’ effluents by clay: adsorption of anionic dye on kaolinite. Arab J Geosci 10, 373 (2017). https://doi.org/10.1007/s12517-017-3161-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-017-3161-3