Abstract
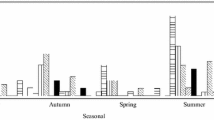
The contribution of combined sewer overflows (CSO) to the viral contamination of receiving waters was determined. Adenovirus concentrations were determined using the Primary Liver Carcinoma (PLC/PRF/5) cell line and confirmed by Polymerase Chain Reaction (PCR). Norovirus concentration was determined using the Most Probable Number (MPN) and Reverse Transcription-Polymerase Chain Reaction (RT-PCR). Seventy-five water samples were collected during dry weather and 50 samples were collected during wet weather. CSO events significantly increased the concentration of culturable viruses, adenoviruses, and noroviruses in the receiving waters (P < 0.01). During dry weather, 56% of samples were positive for total virus cytopathic effects (CPE), adenoviruses were detected in 41% of the positive cell cultures, and noroviruses in 6% of the concentrates by direct RT-PCR. During wet weather, 100% of the samples were positive by CPE, 84% for adenoviruses, and 40% in the concentrates for norovirus. Our results demonstrate that CSOs can contribute significant viral loading to receiving waters.

Similar content being viewed by others
References
Avellón, A., Pérez, P., Aguilar, J. C., Lejarazu, R., & Echevarría, J. E. (2001). Rapid and sensitive diagnosis of human adenovirus infections by a generic polymerase chain reaction. Journal of Virological Methods, 92, 113–120.
Chapron, C. D., Ballester, N. A., Fontaine, J. H., Frades, C. N., & Margolin, A. B. (2000). Detection of astroviruses, enteroviruses, and adenovirus types 40 and 41 in surface waters collected and evaluated by the information collection rule and an integrated cell culture-nested PCR procedure. Applied and Environmental Microbiology, 66, 2520–2525.
Choi, S., & Jiang, S. C. (2005). Real-time PCR quantification of human adenoviruses in urban rivers indicates genome prevalence but low infectivity. Applied and Environmental Microbiology, 71, 7426–7433.
da Silva, A. K., Saux, J., Parnaudeau, S., Pommepuy, M., Elimelech, M., & Le Guyader, F. S. (2007). Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: Different behaviors of genogroups I and II. Applied and Environmental Microbiology, 73, 7891–7897.
Ellis, J. B., & Yu, W. (1995). Bacteriology of urban runoff—the combined sewer as a bacterial reactor and generator. Water Science and Technology, 31, 303–310.
Even, S., Mouchel, J. M., Servais, P., Flipo, N., Poulin, M., Blanc, S., et al. (2007). Modelling the impacts of combined sewer overflows on the river seine water quality. Science of The Total Environment, 375, 140–151.
Fankhauser, R. L., Monroe, S. S., Noel, J. S., Humphrey, C. D., Bresee, J. S., Parashar, U. D., et al. (2002). Epidemiologic and molecular trends of “Norwalk-Like viruses” associated with outbreaks of gastroenteritis in the United States. Journal of Infectious Diseases, 186, 1–7.
Ferguson, C. M., Coote, B. G., Ashbolt, N. J., & Stevenson, I. M. (1996). Relationships between indicators, pathogens and water quality in an estuarine system. Water Research, 30, 2045–2054.
Fong, T. T., Griffin, D. W., & Lipp, E. K. (2005). Molecular assays for targeting human and bovine enteric viruses in coastal waters and their application for library-independent source tracking. Applied and Environmental Microbiology, 71, 2070–2078.
Gerba, C. P. (2009). Wastewater treatment and biosolids reuse. In R. M. Maier, I. L. Pepper, & C. P. Gerba (Eds.), Environmental microbiology (2nd ed., pp. 503–530). San Diego: Academic Press.
Grabow, W. O., Puttergill, D. L., & Bosch, A. (1992). Propagation of adenovirus types 40 and 41 in the PLC/PRF/5 primary liver carcinoma cell line. Journal of Virological Methods, 37, 201–207.
Grabow, W., Botma, K. L., Villiers, J. C., Clay, C. G., & Erasmus, B. (1999). Assessment of cell culture and polymerase chain reaction procedures for the detection of poliovirus in wastewater. Bulletin—World Health Organisation, 77, 973–978.
Haramoto, E., Katayama, H., Oguna, K., Yamashita, H., Tarima, A., Nakajima, H., et al. (2006). Seasonal profiles of human norovirues and indicator bacteria in the wastewater treatment plant in Tokyo, Japan. Water Science and Technology, 54, 301–308.
He, J. W., & Jiang, S. (2005). Quantification of enterococci and human adenoviruses in environmental samples by real-time PCR. Applied and Environmental Microbiology, 71, 2250–2255.
Hurley, M. A., & Roscoe, M. E. (1983). Automated statistical-analysis of microbial enumeration by dilution series. Journal of Applied Bacteriology, 55, 159–164.
Jiang, S. C. (2006). Human adenoviruses in water: occurrence and health implications: A critical review. Environmental Science Technology, 40, 7132–7140.
Jiang, S. C., & Chu, W. (2004). PCR detection of pathogenic viruses in southern Califonia urban rivers. Journal of Applied Microbiology, 97, 17–28.
Karim, M. R., & LeChevallier, M. W. (2004). Detection of noroviruses in water: Current status and future directions. Journal of Water Supply: Research and Technology-AQUA, 53, 359–380.
Katayama, H., Okuma, K., Furumai, H., & Ohgaki, S. (2004). Series of surveys for enteric viruses and indicator organisms in Tokyo Bay after an event of combined sewer overflow. Water Science and Technology, 50, 259–262.
Katayama, H., Haramoto, E., Oguma, K., Yamashita, H., Tajiama, A., Nakajima, H., et al. (2008). One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Research, 42, 1441–1448.
Maloney, A. (2007). Comparison of conventional and taqman real-time RT-PCR assay for the detection of norovirus. MS thesis, University of North Carolina at Chapel Hill.
Rijal, G., Petropoulou, C., Tolson, J. K., DeFlaun, M., Gerba, C., Gore, R., et al. (2009). Dry and wet weather microbial characterization of the Chicago area waterway system. Water Science and Technology, 60, 1847–1855.
Rijal, G., Tolson, J. K., Petropoulou, C., Granato, T., Glymph, T., DeFlaun, M., et al. (2011). Microbial risk assessment for recreational use of the Chicago area waterway system. Journal of Water and Health, 9(1), 169–186.
Rodriguez, R. A., Gundy, P. M., & Gerba, C. P. (2008). Comparison of BGM and PLC/PRC/5 cell lines for total culturable viral assay of treated sewage. Applied and Environmental Microbiology, 74, 2583–2587.
Rose, J. B., Dickson, L. J., Farrah, S. R., & Carnahan, R. P. (1996). Removal of pathogenic and indicator microorganisms by a full-scale water reclamation facility. Water Research, 30, 2785–2797.
Sedmak, G., Bina, D., McDonald, J., & Couillard, L. (2005). Nine-year study of the occurrence of culturable viruses in source water for two drinking water treatment plants and the influent and effluent of a wastewater treatment plant in Milwaukee, Wisconsin (August 1994 Through July 2003). Applied and Environmental Microbiology, 71, 1042–1050.
Straub, T. M., Höner zu Bentrup, K., Orosz-Coghlan, P., Dohnalkova, A., Mayer, B. K., Bartholomew, R. A., et al. (2007). In vitro cell culture infectivity assay for human noroviruses. Emerging Infectious Diseases, 13, 396–403.
Thompson, S. S., Jackson, J. L., Suva-Castillo, M., Yanko, W. A., El Jack, Z., Kuo, J., et al. (2003). Detection of infectious human adenoviruses in tertiary-treated and ultraviolet-disinfected wastewater. Water Environment Research, 75, 163–170.
Thurston-Enriquez, J. A., Haas, C. N., Jacangelo, J., Riley, K., & Gerba, C. P. (2003). Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Applied and Environmental Microbiology, 69, 577–582.
USEPA. (2001a). Concentration and processing of waterborne viruses by positive charge 1MDS cartridge filters and organic flocculation, Chapter 14. In USEPA manual of methods for virology. Washington DC: EPA .Office of research and development.
USEPA. (2001b). Total culturable virus quantal assay, Chapter 15. In USEPA manual of methods for virology. Washington DC: EPA Office of research and development.
Vinjé, J., Hamidjaja, R. A., & Sobsey, M. D. (2004). Development and application of a capsid Vp1 (Region D) based reverse transcription PCR assay for genotyping of genogroup I and II noroviruses. Journal of Virological Methods, 116, 109–117.
Ward, R. L., Knowlton, D. R., & Pierce, M. J. (1986). Mechanisms of inactivation of enteric viruses in fresh waters. Applied and Environmental Microbiology, 52, 450–459.
Wojtenko, I., Stinson, M. K., & Field, R. (2001a). Challenges of combined sewer overflow disinfection by ultraviolet light irradiation. Critical Reviews in Environmental Science and Technology, 31, 223–239.
Wojtenko, I., Stinson, M. K., & Field, R. (2001b). Performance of ozone as a disinfectant for combined sewer overflow. Critical Reviews in Environmental Science and Technology, 31, 295–309.
Acknowledgments
Special thanks to Francisco Calderon and Sara Beck for their comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez, R.A., Gundy, P.M., Rijal, G.K. et al. The Impact of Combined Sewage Overflows on the Viral Contamination of Receiving Waters. Food Environ Virol 4, 34–40 (2012). https://doi.org/10.1007/s12560-011-9076-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-011-9076-3