Abstract
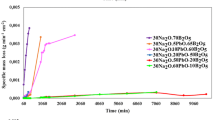
The borosilicate glass is the most common industrial solution host barrier used for the immobilization of low-level wastes for its excellent chemical durability and mechanical properties. The borosilicate glass samples were prepared in a muffle furnace at a temperature 1300 °C, glass samples were then annealed in a furnace at 400 °C. In this study, density, microhardness, pH and the durability of borosilicate glass were tested. Many leaching solutions such as HCl, NaOH and ground water were used. The results show that the prepared glass has good microhardness. The glass composition containing 37.5 % slag −37.5 % cement dust – 25 % B2O3 is the most durable glass in acidic solution and the borosilicate glass of the composition 37.5 % cullet – 37.5 % cement dust – 25 % B2O3 is the most corroded glass in acidic solution. The durability was also studied after subjecting the glass samples to different irradiation doses of γ- rays. Infrared absorption results were interpreted in the light of the effect of corrosion and irradiation on the vibrational bands.
Similar content being viewed by others
References
Sheng J (2001) Vitrification of borate waste from nuclear power plant using coal fly ash. (1) Glass formulation development. J Fuel:1365–1369
Ojovan MI, Lee WE (2013) Fundamentals of radioactive waste (RAW): science, sources, classification and management strategies. J Radioactive waste management and contained site clean- up:3–49
Sobolev IA, Dmitiev SA, Lijanov FA, Kobelev AP, Stepnoveky SV, Ojovan MI (2005) Vitrification processes for low, intermediate radioactive and mixed wastes. Glass Technol-European Journal of Glass Science and Technology Part A:28–35
Colombo P, Brusatin G, Bernardo E, Scarinci G (2003) Inertization and reuse of waste materials by vitrification and fabrication of glass-based products. Cun Opn Solid State Mater Sci:225–239
Jouan A (2001) Waste vitrification: a contribution to safeguarding the earth. Proc Int Cong on Glass, Edinburg, pp 286–291
Sheng J, Luo S (2001) 90-19/U HLW-glass leaching mechanism in underground water. J Nucl Mater:57–61
Akai T, Kuraoka K, Chen D, Yamamato Y, Shirakame T, Urabe K (2005) Leaching behavior of sodium from fine particles of soda-lime-silicate glass in acid solution. J Amer Ceram Soc:2962–2965
El-Alaily NA, Mahmoud HH, Abd-Elaziz TD, Ezz-eldin FM (2011) Effect of concentration and irradiation on the etching of soda-lime-silica glass. Aust J Basic Appl Sci:186–196
Doremus RH (1994) Glass Science” 2nd ed. Wiley Inter science publications, NY
Scholze H (1991) Glass nature, structure and properties. Springer, NY
El-Alaily NA, Ezz-Eldin FM, El-Batal HA (1997) Effect of gamma irradiation on the corrosion of some lead-silicate glasses. J Nucl Sci:164–1
El-Batal HA, Azooz MA, Ezz-Eldin FM, El-Alaily NA (2001) Interaction of gamma-rays with calcium aluminoborate glasses containing holmium or erbuim. J Amer Ceram Soc:2065–2072
El-Alaily NA, Rafaat EA, Mahmoud HH, Gonaim AKh, Abd-elaziz TD, Hamed E, Ezz-Eldin FM (2007) Effect of different aqueous solutions on γ irradiated commercial and simulated soda lime silicate glass. J Egypt Chem:645–666
El-Alaily NA, Ezz-Eldin FM (2003) Immobilization of radioactive waste in boro-silicate or cabal glasses. J Arab Nucl Sci Appl:63–73
Haddi A, Trocellier P, Dragomic I, Farges F, Poissonnet S, Bonnaillie P (2008) Ion irradiation behavior and chemical durability of simple borosilicate glasses. J Nucl Inst Methods Phys Res:3182–3185
Ojovan MI, Lee WE (2004) Alkali ion exchange in gamma-irradiated glasses. J Nucl Mater:425–32
Skuja L (1998) Optically active oxygen-deficiency-related centers in amorphous silicon dioxide. J Non Cryst Solids:16–48
Weber WJ, Ewing RC, Angel CA, Arnold GW, Cormack AN, Delaye DL, Griscom LW, Hobbs LW, Navrotsky A, Price DL, Stomeham AM, Weinberg MC (1997) Radiation effects in glasses used for immobilizing of high level waste and plutonium disposition. J Mater Res:1948–1987
Clarida WJ, Berryman JR, Affatigato M, Feller SA, Kroeker S, Ash J, Zwanzinger JW, Meyer BM, Borsa F, Martin SW (2003) Dependence of N4 upon alkali modifier in binary borate glasses. Phys Chem Glasses:215–217
El-Alaily NA (2003) Study of some properties of lithium silicate glass and glass ceramics containing blast furnace slag. Glass Technol 44:30–38
Ezz El-Din FM, ElAlaily NA, El-Batal HA (1992) Density and of refractive index some gamma-irradiated alkali silicate glasses. J Radiat Nucl Chem:267–275
Primak W (1979) The x-ray compaction of vitreous silica. J Rad Effects:29–32
McDonald RS (1958) Surface functionality of amorphous silica by infrared spectroscopy. J Phys Chem 62:1168–1178
ElBatal FH, Azooz MA, Hamdy YM, Ezz-Eldin FM (2010) Characterization and gamma irradiation studies of glass ceramics derivatives obtained from blast urance slag for immobliziation of radioactive wastes. Transactions Indian Ceram Soc:29–36
Rao TGVM, Rupesh Kumar A, Neeraja K, Veeraiah N, Rami Reddy M (2013) Optical and structural investigation of Eu3+ ions in Nd3+ co-doped magnesium lead borosilicate glasses. J Alloys Compd:209–217
Rupesh Kumar A, Rao TGVM, Neeraja K, Rami Reddy M, Veeraiah N (2013) Gamma ray induced changes on vibrational spectroscopic propertiesof strontium alumino-borosilicate glasses. Vib Spectrosc:49–56
Stoch L, Sroda M (1999) Infrared spectroscopy investigation of oxide glasses structure. J Mol Struct:77–84
Kansal I, Goel A, Tulyaganov DU, Shaaban ER, Ribeiro MJ, Ferreira JMF (2009) Ceram Int 35:3013–3019
Merzbacher CI, White WB (1991) The structure of alkaline earth aluminosilicate glasses as determined by vibrational spectroscopy. J Non Cryst Solids 130:18–34
Kamitsos EI (2003) Infrared studies of borate glasse. Phys Chem Glasses 44:79–78
Dunken H, Doremus RH (1987) Short time reactions of A Na,O-Cao-Sio, glass with water and salt solutions. J Non-Cryst Solids:61–72
Clark DE, Pantano CG, Hench LL (1979) Corrosion of glass. Book for industry, NY
Ernsberger FM (1977) Molecular water in glass. J Am ceram:91–92
Manupriya K, Thind S, Sharma G, Singh K (2007) Soluble borate glasses: in vitro analysis. J Amer Ceram Soc 90:467–471
Jedlicka AB, Clare AG (2001) Chemical durability of commercial silicate glasses. I. Material characterization. J Non Cryst Solids 281:6–24
Ahmed AA, Abbas AF, Youssof IM (1995) Attack of lead crystal glass by aqueous solution of ethyl and methyl alcohol. Proceedings XVII International Congress on glass, vol 3. Chinese Ceramic Society, Beijing, pp 239–44
Perera G, Doremus R (1991) Dissolution rates of commercial soda-lime and pyrex borosilicate glasses: influence of solution pH. J Am Ceram Soc 74:1269–1274
Salem AA, Kellner R, Grasserbauer M (1994) Corrosion processes in glass by a multitechnique approach. 2. Infrared- Spectroscopy. Glass Technol 35:135–140
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Alaily, N.A., Abdallah, W.M., Sabrah, B.A. et al. Preparation and Characterization of Immobilizing Radioactive Waste Glass From Industrial Wastes. Silicon 9, 117–130 (2017). https://doi.org/10.1007/s12633-015-9330-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-015-9330-7