Abstract
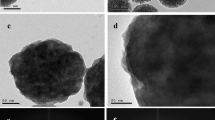
Magnesium calcium silicate nanostructures (MCSNS) loaded with (0.0, 0.6, 0.9, and 1.2 wt%) of Cephradine-drug consisting of mesoporous particles were functionally prepared by sol-gel method and treated at 60oC. Bio-functionally nanostructures were characterized by XRD, SEM, FTIR, diffuse reflectance, UV-Vis absorbance and antimicrobial experiments. MCSNS/Velosef nanostructures showed highly successful loading for Cephradine within the silicate-based matrix. As the concentration of velosef increases in the nanostructure, the optical bandgap decreases. The bactericidal features of prepared xerogel nanodrugs against E.coli and Staphylococcus aureus were investigated. The results reveal that these nanoparticles have a powerful bactericidal ability against the tested bacterial strains. The antibacterial properties of all nanomedicines against E. coli were greater than S.aureus. Overall, the results obtained in this study concluded that MCS loaded with 1.2 wt% Cephradine nanostructures could be a powerful bactericidal agent for the elimination of the emerging nosocomial pathogens. Further, this structure recognizes the conceivable manner for applying in biomedical and pharmaceutical applications.
Similar content being viewed by others
References
Xin Q, Shah H, Nawaz A et al (2019) Antibacterial Carbon-Based Nanomaterials. Adv Mater 31:1804838. https://doi.org/10.1002/adma.201804838
Khan HA, Baig FK, Mehboob R (2017) Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed 7:478–482. https://doi.org/10.1016/j.apjtb.2017.01.019
El Malah T, Nour HF, Satti AAE et al (2020) Design, synthesis, and antimicrobial activities of 1,2,3-triazole glycoside clickamers. Molecules 25:790. https://doi.org/10.3390/molecules25040790
Siddique T, Farzand S, Waheed SS, Khan F (2012) Frequency and etiology of nosocomial infections in medical unit-I, nawaz sharif social security teaching hospital Lahore. Pak J Med Health Sci 6:499–501
Shoaei S, Sali S, Yousefi H (2017) Incidence and resistance patterns of nosocomial infections in Labbafi Nejad Hospital admitted patients during 2012–2014. Infect Epidemiol Microbiol 3:78–81
Argudín M, Deplano A, Meghraoui A et al (2017) Bacteria from animals as a pool of antimicrobial resistance genes. Antibiotics 6:12. https://doi.org/10.3390/antibiotics6020012
Radwan MAA, Alshubramy MA, Abdel-Motaal M et al (2020) Synthesis, molecular docking and antimicrobial activity of new fused pyrimidine and pyridine derivatives. Bioorg Chem 96:103516. https://doi.org/10.1016/j.bioorg.2019.103516
Hasanova UA, Vezirova LZ, Gakhramanova ZO (2018) Synthesisof nanostructures on the basis of diazacrown ether and magnetite nanoparticles loaded by cephalosporin antibiotics. Azerbaijan Chem J 3:66–73. https://doi.org/10.32737/0005-2531-2018-3-66-73
Hemeg H (2017) Nanomaterials for alternative antibacterial therapy. Int J Nanomed 12:8211–8225. https://doi.org/10.2147/IJN.S132163
Etheridge ML, Campbell SA, Erdman AG et al (2013) The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomed Nanotechnol Biol Med 9:1–14
Drnovšek N, Novak S, Dragin U et al (2012) Bioactive glass enhances bone ingrowth into the porous titanium coating on orthopaedic implants. Int Orthop 36:1739–1745. https://doi.org/10.1007/s00264-012-1520-y
Liu L, Chen S (2015) Theoretical study on cyclopeptides as the nanocarriers for Li +, Na +, K + and F –, Cl –, Br –. J Nanomater 2015:1–7. https://doi.org/10.1155/2015/276191
Song M, Li Y, Ning A et al (2010) Silica nanoparticles as a carrier in the controlled release of florfenicol. J Drug Deliv Sci Technol 20:349–352. https://doi.org/10.1016/S1773-2247(10)50058-3
ElNahrawy AM, AbouHammad AB (2016) A facile co-gelation sol gel route to synthesize cao: P2o5: Sio2 xerogel embedded in chitosan nanocomposite for bioapplications. Int J PharmTech Res 9:16–21
Abou Hammad AB, Elnahrawy AM, Youssef AM, Youssef AM (2019) Sol gel synthesis of hybrid chitosan/calcium aluminosilicate nanocomposite membranes and its application as support for CO2 sensor. Int J Biol Macromol 125:503–509. https://doi.org/10.1016/j.ijbiomac.2018.12.077
Choudhary R, Koppala S, Swamiappan S (2015) Bioactivity studies of calcium magnesium silicate prepared from eggshell waste by sol–gel combustion synthesis. J Asian Ceam Soc 3:173–177. https://doi.org/10.1016/j.jascer.2015.01.002
Sánchez-Salcedo S, Shruti S, Salinas AJ et al (2014) In vitro antibacterial capacity and cytocompatibility of SiO 2-CaO-P2O5 meso-macroporous glass scaffolds enriched with ZnO. J Mater Chem B 2:4836–4847. https://doi.org/10.1039/c4tb00403e
Rahaman MN, Day DE, Sonny Bal B et al (2011) Bioactive glass in tissue engineering. Acta Biomater 7:2355–2373. https://doi.org/10.1016/j.actbio.2011.03.016
Hench LL (1991) Bioceramics: From concept to clinic. J Am Ceram Soc 74:1487–1510. https://doi.org/10.1111/j.1151-2916.1991.tb07132.x
Yang Y, Yang RB, Fan HJ et al (2010) Diffusion-facilitated fabrication of gold-decorated Zn2SiO4 nanotubes by a one-step solid-state reaction. Angew Chem Int Ed 49:1442–1446. https://doi.org/10.1002/anie.200906022
Zhuang Y, Yang Y, Xiang G, Wang X (2009) Magnesium silicate hollow nanostructures as highly efficient absorbents for toxic metal ions. J Phys Chem C 113:10441–10445. https://doi.org/10.1021/jp9014756
Denning DW, Perlin DS, Muldoon EG et al (2017) Delivering on antimicrobial resistance agenda not possible without improving fungal diagnostic capabilities. Emerg Infect Dis 23:177–183. https://doi.org/10.3201/eid2302.152042
van den Broek LAM, Knoop RJI, Kappen FHJ, Boeriu CG (2015) Chitosan films and blends for packaging material. Carbohyd Polym 116:237–242. https://doi.org/10.1016/j.carbpol.2014.07.039
Carlisle EM (1980) Biochemical and morphological changes associated with long bone abnormalities in silicon deficiency. J Nutr 110:1046–1056. https://doi.org/10.1093/jn/110.5.1046
Wu C, Chang J (2007) Degradation, bioactivity, and cytocompatibility of diopside, akermanite, and bredigite ceramics. J Biomed Mater Res B Appl Biomater 83B:153–160. https://doi.org/10.1002/jbm.b.30779
Taxak VB, Khatkar SP, Kumar M, Han S-D (2019) Combustion synthesis and photoluminescence characteristics of Y 1-x CaAl 3 O 7:xEu 3 + nanoparticles. ECS Trans 28:155–160. https://doi.org/10.1149/1.3367221
Ventura JMG, Tulyaganov DU, Agathopoulos S, Ferreira JMF (2006) Sintering and crystallization of akermanite-based glass–ceramics. Mater Lett 60:1488–1491. https://doi.org/10.1016/j.matlet.2005.11.059
Wu C, Chang J (2004) Synthesis and apatite-formation ability of akermanite. Mater Lett 58:2415–2417. https://doi.org/10.1016/j.matlet.2004.02.039
Wu C, Zhang M, Zhai D et al (2013) Containerless processing for preparation of akermanite bioceramic spheres with homogeneous structure, tailored bioactivity and degradation. J Mater Chem B 1:1019–1026. https://doi.org/10.1039/C2TB00215A
Kortesuo P, Ahola M, Karlsson S et al (1999) Sol-gel-processed sintered silica xerogel as a carrier in controlled drug delivery. J Biomed Mater Res 44:162–167. https://doi.org/10.1002/(SICI)1097-4636(199902)44:2<162::AID-JBM6>3.0.CO;2-P
Ahola M, Kortesuo P, Kangasniemi I et al (2000) Silica xerogel carrier material for controlled release of toremifene citrate. Int J Pharm 195:219–227. https://doi.org/10.1016/S0378-5173(99)00403-2
El Nahrawy AM, Hemdan BA, Abou Hammad AB et al (2019) Microstructure and antimicrobial properties of bioactive cobalt co-doped copper aluminosilicate nanocrystallines. Silicon. https://doi.org/10.1007/s12633-019-00326-y
El Nahrawy AM, Salah El-Deen H, Soliman AA, Mosa WMM (2018) Crystallographic and magnetic properties of Al3 + co-doped NiZnFe2O4 nano- particles prepared by sol-gel process. Egypt J Chem 62:525–532. https://doi.org/10.21608/ejchem.2018.4504.1397
Kortesuo P, Ahola M, Karlsson S et al (2000) Silica xerogel as an implantable carrier for controlled drug delivery—evaluation of drug distribution and tissue effects after implantation. Biomaterials 21:193–198. https://doi.org/10.1016/S0142-9612(99)00148-9
Ahola MS, Säilynoja ES, Raitavuo MH et al (2001) In vitro release of heparin from silica xerogels. Biomaterials 22:2163–2170. https://doi.org/10.1016/S0142-9612(00)00407-5
Radin S, Chen T, Ducheyne P (2009) The controlled release of drugs from emulsified, sol gel processed silica microspheres. Biomaterials 30:850–858. https://doi.org/10.1016/j.biomaterials.2008.09.066
Barbé C, Bartlett J, Kong L et al (2004) Silica particles: A novel drug-delivery system. Adv Mater 16:1959–1966. https://doi.org/10.1002/adma.200400771
Otsuka M, Tokumitsu K, Matsuda Y (2000) Solid dosage form preparations from oily medicines and their drug release. Effect of degree of surface-modification of silica gel on the drug release from phytonadione-loaded silica gels. J Control Release 67:369–384. https://doi.org/10.1016/S0168-3659(00)00229-7
Tang Q, Xu Y, Wu D, Sun Y (2006) A study of carboxylic-modified mesoporous silica in controlled delivery for drug famotidine. J Solid State Chem 179:1513–1520. https://doi.org/10.1016/j.jssc.2006.02.004
Charnay C, Bégu S, Tourné-Péteilh C et al (2004) Inclusion of ibuprofen in mesoporous templated silica: drug loading and release property. Eur J Pharm Biopharm 57:533–540. https://doi.org/10.1016/j.ejpb.2003.12.007
El-Nahrawy AM, Ali AI, Abou Hammad AB, Youssef AM (2016) Influences of Ag-NPs doping chitosan/calcium silicate nanocomposites for optical and antibacterial activity. Int J Biol Macromol 93:267–275. https://doi.org/10.1016/j.ijbiomac.2016.08.045
Ali GW, El-Hotaby W, Hemdan B, Abdel-Fattah WI (2018) Thermosensitive chitosan/phosphate hydrogel-composites fortified with Ag versus Ag@Pd for biomedical applications. Life Sci 194:185–195. https://doi.org/10.1016/j.lfs.2017.12.021
Gaballah ST, El-Nazer HA, Abdel-Monem RA et al (2019) Synthesis of novel chitosan-PVC conjugates encompassing Ag nanoparticles as antibacterial polymers for biomedical applications. Int J Biol Macromol 121:707–717. https://doi.org/10.1016/j.ijbiomac.2018.10.085
Lavorgna M, Iacovino R, Russo C et al (2019) A new approach for improving the antibacterial and tumor cytotoxic activities of pipemidic acid by including it in Trimethyl-β-cyclodextrin. Int J Mol Sci 20:416. https://doi.org/10.3390/ijms20020416
Hemdan BA, El Nahrawy AM, Mansour A-FM, Hammad ABA (2019) Green sol–gel synthesis of novel nanoporous copper aluminosilicate for the eradication of pathogenic microbes in drinking water and wastewater treatment. Environ Sci Pollut Res 26:9508–9523. https://doi.org/10.1007/s11356-019-04431-8
Nakamoto K (2008) Infrared and raman spectra of inorganic and coordination compounds. Wiley, Hoboken
Nakamoto K (2009) Applications in inorganic chemistry. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Wiley, Hoboken, pp 149–354
Kalinkin AM, Boldyrev VV, Politov AA et al (2003) Investigation into the mechanism of interaction of calcium and magnesium silicates with carbon dioxide in the course of mechanical activation. In: Glass Physics and Chemistry. pp 410–414
Masri A, Anwar A, Ahmed D et al (2018) Silver nanoparticle conjugation-enhanced antibacterial efficacy of clinically approved drugs cephradine and vildagliptin. Antibiotics 7:100. https://doi.org/10.3390/antibiotics7040100
Yang L, Kruse B (2004) Revised Kubelka–Munk theory I theory and application. J Opt Soc Am A. https://doi.org/10.1364/JOSAA.21.001933
Mansour AM (2019) Fabrication and characterization of a photodiode based on 5′,5′′-dibromo-o-cresolsulfophthalein (BCP). Silicon 11:1989–1996. https://doi.org/10.1007/s12633-018-0016-9
Farag AAM, Mansour AM, Ammar AH et al (2012) Electrical conductivity, dielectric properties and optical absorption of organic based nanocrystalline sodium copper chlorophyllin for photodiode application. J Alloy Compd 513:404–413. https://doi.org/10.1016/j.jallcom.2011.10.058
Al-Khodir FAI, Refat MS (2017) Physicochemical, spectroscopic, and anti-tumor studies of cefradine complexes with Ca(II), Zn(II), Fe(III), Au(III), and Pd(II) ions. Russ J Gen Chem 87:1087–1092. https://doi.org/10.1134/S1070363217050322
Guo X, Wu J, Yiu Y-M et al (2013) Drug–nanocarrier interaction—tracking the local structure of calcium silicate upon ibuprofen loading with X-ray absorption near edge structure (XANES). Phys Chem Chem Phys 15:15033. https://doi.org/10.1039/c3cp50699a
Amann S, Neef K, Kohl S (2019) Antimicrobial resistance (AMR). Eur J Hosp Pharm 26:175–177. https://doi.org/10.1136/ejhpharm-2018-001820
Zhu YJ, Sham TK (2014) The potential of calcium silicate hydrate as a carrier of ibuprofen. Expert Opin Drug Deliv 11:1337–1342. https://doi.org/10.1517/17425247.2014.923399
Huang C-Y, Huang T-H, Kao C-T et al (2017) Mesoporous calcium silicate nanoparticles with drug delivery and odontogenesis properties. J Endod 43:69–76. https://doi.org/10.1016/j.joen.2016.09.012
Ahmad V, Khan MS, Jamal QMS et al (2017) Antimicrobial potential of bacteriocins: in therapy, agriculture and food preservation. Int J Antimicrob Agents 49:1–11. https://doi.org/10.1016/j.ijantimicag.2016.08.016
Chen F, Zhu Y, Wu J et al (2012) Nanostructured calcium phosphates: Preparation and their application in biomedicine. Nano Biomed Eng 4:41–49. https://doi.org/10.5101/nbe.v4i1.p41-49
Ebrahimi M, Botelho MG, Dorozhkin SV (2017) Biphasic calcium phosphates bioceramics (HA/TCP): Concept, physicochemical properties and the impact of standardization of study protocols in biomaterials research. Mater Sci Eng C 71:1293–1312. https://doi.org/10.1016/j.msec.2016.11.039
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Nahrawy, A.M., Hemdan, B.A., Abou Hammad, A.B. et al. Modern Template Design and Biological Evaluation of Cephradine-loaded Magnesium Calcium Silicate Nanocomposites as an Inhibitor for Nosocomial Bacteria in Biomedical Applications. Silicon 13, 2979–2991 (2021). https://doi.org/10.1007/s12633-020-00642-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-020-00642-8