Abstract
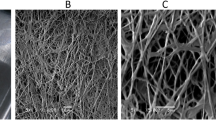
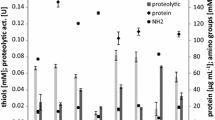
Response surface methodology was employed for optimization of the culture conditions for keratinase production by a feather-degrading Paenibacillus woosongensis TKB2 under solid-state fermentation using chicken feather as substrate. The optimized culture conditions having 2.21 g of feather, with rice straw (2:1), moistened with distilled water (1:5, w/v adjusted to pH 8.5) and fermented for 72.07 h, resulted in 2.83-fold increased keratinase production compared to non-optimized condition. The enzyme efficiently dehaired goat hides within 14 h without the use of lime. The enzyme-dehaired goat skins were evaluated by mechanical, touch-visual and electron microscopy methods and were compared with conventional chemical treated skins. The results indicated that the enzyme treated dehaired skins exhibit similar or improved characteristics without damaging the collagen layer, which makes the crude keratinase a potential candidate for application in leather industry to avoid pollution problems associated with the use of chemicals.





Similar content being viewed by others
References
Onifade, A.A., Sane, N.A., Musallam, A.A.A., Zarban, S.A.: A review: potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour. Technol. 66, 1–11 (1998)
Gupta, R., Rammani, P.: Microbial keratinases and their prospective applications: an overview. Appl. Microbiol. Biotechnol. 7, 21–33 (2006)
Brandelli, A.: Bacterial keratinases: useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioprocess Technol. 1, 105–116 (2008)
Shih, J.C.H.: Recent developments in poultry waste digestion and feather utilization. Poul. Sci. 72, 1617–1620 (1993)
Rai, S.K., Konwarh, R., Mukherjee, A.K.: Purification, characterization and biotechnological application of an alkaline β-keratinase produced by Bacillus subtilis RM-01 in solid-state fermentation using chicken-feather as substrate. Biochem. Eng. J. 45, 218–225 (2009)
Kida, K., Morimura, S., Noda, J., Nishida, Y., Imai, T., Otagiri, M.J.: Enzymatic hydrolysis of the horn and hoof of cow and buffalo. J. Ferment. Bioeng. 80(5), 478–484 (1995)
Martignone, G., Monteverdi, R., Roaldi, M., Galante, Y.M.: Progress for leather makers. Leather 199, 35–40 (1997)
Addy, V., Garwood, R.: Microscopy problem solving, World Leather. 94–100 (1997)
Thanikaivelan, P., Rao, J.R., Nair, B.U.: Development of a leather processing method in narrow pH profile. Part1. Standardisation of unhairing process. J. Soc. Leather Technol. Chem. 84, 276–284 (2000)
Valeika, V., Beleska, K., Valeikiene, V., Kolodzeiskiset, W.: An approach to cleaner production: from hair burning to hair saving using a lime-free unhairing system. J. Clean. Prod. 17, 214–222 (2009)
Jian, S., Wenyi, T., Wuyong, C.: Kinetics of enzymatic unhairing by protease in leather industry. J. Clean. Prod. 19, 325–331 (2011)
Paul, T., Halder, S.K., Das, A., Bera, S., Maity, C., Mandal, A., Das, P.S., Mohapatra, P.K.D., Pati, B.R., Mondal, K.C.: Exploitation of chicken feather waste as a plant growth promoting agent using keratinase producing novel isolate Paenibacillus woosongensis TKB2. Biocat. Argricul. Biotechnol. 2, 50–57 (2013). doi:10.1016/j.bcab.2012.10.001. (Article in press)
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J.: Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 267–275 (1951)
Prophet, E.B., Mills, B., Arrington, J.B., Sobin, L.H.: Laboratory Methods in Histotechnology. Armed Forces Institute of Pathology, p. 279. American Registry of Pathology, Washington, DC (1992)
Gidanian, S., Farmer, P.J., Meyskens, F.L.: Melanin oxidation and possible relation to melanosomal abnormalities. Pigment Cell Res. 17, 446 (2004)
Clesceri, L.S., Greenberg, A.E., Trussell, R.R.: Standard methods: for the estimation of water and wastewater, 17th edn. American Public Health Association, Washington, DC (1989)
Madhan, B., Dineshkumar, M., Rao, J.R., Nair, B.U.: Studies on the removal of inter-fibrillary materials part I: removal of protein, proteoglycan and glycosoaminoglycans from conventional beam house process. JALCA 105, 145–149 (2010)
Dettmer, A., Nunes, K.G.P., Gutterres, M., Marcílio, N.R.: Obtaining sodium chromate from ash produced by thermal treatment of leather wastes. Chem. Eng. J. 160, 8–12 (2010)
Kar, S., Gauri, S.S., Das, A., Jana, A., Maity, C., Mandal, A., Das Mohapatra, P.K., Pati, B.R., Mondal, K.C.: Process optimization of xylanase production using cheap solid substrate by Trichodermareesei SAF3 and study on the alteration of behavioral properties of enzyme obtained from SSF and SmF. Bioprocess Biosyst. Eng. 36, 57–68 (2013)
Balaji, S., Senthil, K.M., Karthikeyan, R., Ramadhar, K.S., Kirubanandan, R., Sridhar Sehgal, P.K.: Purification and characterization of an extracellular keratinase from a horn meal-degrading Bacillus subtilis MTCC (9102). World J. Microbiol. Biotechnol. 24, 2741–2745 (2008)
Nilegaonkar, S.S., Zambare, V.P., Kanekar, P.P., Dhakephalkar, P.K., Sarnaik, S.S.: Production and partial characterization of dehairing protease from Bacillus cereus MCM B-326. Bioresour. Technol. 98, 1238–1245 (2007)
Boer, C.G., Peralta, R.M.: Production of extracellular protease by Aspergillus tamari. J. Basic Microbiol. 40, 75–81 (2000)
Ismail, A.M.S., Housseiny, M.M., Abo-Elmagd, H.I., El-Sayed, N.H., Habib, M.: Novel keratinase from Trichodermaharzianum MH-20 exhibiting remarkable dehairing capabilities. Int. Biodeterior. Biodegrad 70, 14–19 (2012)
Kandasamy, N., Punitha, V., Amsamani, S., Rao, J.R., Chandrasekaran, B., Thanikaivelan, P.: Eco-benign enzymatic dehairing of goatskins utilizing a protease from a Pseudomonas fluorescens species isolated from fish visceral waste. J. Clean. Prod. 25, 27–33 (2012)
Nashy, E.H.A., Ismail, S.A., Ahmady, A.M., Fadaly, H.E., Sayed, N.H.: Enzymatic bacterial dehairing of bovine hide by a locallyisolated strain of Bacillus licheniformis. J. Soc. Leath. Technol. Chem. 89, 242–249 (2005)
Senthilvelan, T., Kanagaraj, J., Mandal, A.B.: Application of enzymes for dehairing of skins: cleaner leather processing. Clean. Technol. Environ. Policy. (2012). doi:10.1007/s10098-012-0458-5. (Article in press)
Stark, K.B., Gallas, J.M., Zajac, G.W., Eisner, M., Golab, J.T.: Spectroscopic study and simulation from recent structural models for eumelanin: I. Monomer, dimers. J. Phys. Chem. B 107, 3061–3067 (2003)
Tran, M.L., Powell, B.J., Meredith, P.: Chemical and structural disorder in eumelanins: a possible explanation for broadband absorbance. Biophys. J. 90, 743 (2006)
Acknowledgments
The authors are thankful to the University Grant Commission (UGC), New Delhi for financial support by providing RFSMS Fellowship. We are also very much thankful to Dr. Santi Mohan Mandal, IIT-Khargpur for kind co-operation for providing instrumental facility and Nandadulal Bhattacharyya, VIH, Midnapore for meticulous revision of the paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paul, T., Das, A., Mandal, A. et al. Effective Dehairing Properties of Keratinase from Paenibacillus woosongensis TKB2 Obtained Under Solid State Fermentation. Waste Biomass Valor 5, 97–107 (2014). https://doi.org/10.1007/s12649-013-9217-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-013-9217-z