Abstract
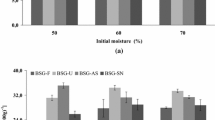
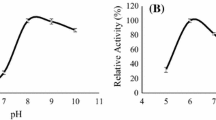
In this study a valuable fermented brewer’s spent grain (BSG) was obtained by solid state fermentation (SSF) with Rhizopus sp. and assessed for feed and food applications. SSF conditions were optimized by factorial design and response surface methodology (RSM) to maximize the value of the resulting BSG biomass. Two Rhizopus sp. strains were tested as inoculum (one wild and one mutant strain) and time and temperature were analyzed. Measured response variables included, among others, protein content, soluble protein, degree of hydrolysis, antioxidant activity, total phenolic content and antibacterial activity. Both strains led to the highest protein concentration (31.7 ± 7.6%) and soluble protein (47.4 ± 3.8 mg/g DM) when BSG was fermented at 30 °C for 9 days. The biomass obtained presented a modified amino acid profile resulting in an essential amino acid index (EAAI) of 1.58 compared to FAO human nutrition standard, with antioxidant capacity (59.7 ± 7.7% DPPH reduction) and 11 times higher total polyphenol content (2.7 ± 0.1 mg GAE/g DM). Hereby, results demonstrate that SSF of BSG results in a significant increase of highly appreciated characteristics for feed or food applications, which could lead to a promising valorization alternative.




Similar content being viewed by others
Abbreviations
- AA:
-
Amino acids
- EAA:
-
Essential amino acids
- EAAI:
-
Essential amino acid index
- FA:
-
Fatty acids
- DH:
-
Degree of hydrolysis
- TEAC:
-
Trolox equivalent antioxidant capacity
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- TPC:
-
Total phenolic content
References
Mussatto, S.I., Dragone, G., Roberto, I.C.: Brewers’ spent grain: generation, characteristics and potential applications. J. Cereal Sci. 43(1), 1–14 (2006). https://doi.org/10.1016/j.jcs.2005.06.001
Santos, M., Jiménez, J.J., Bartolomé, B., Gómez-Cordovés, C., del Nozal, M.J.: Variability of brewer’s spent grain within a brewery. Food Chem. 80(1), 17–21 (2003). https://doi.org/10.1016/S0308-8146(02)00229-7
Steiner, J., Procopio, S., Becker, T.: Brewer’s spent grain: source of value-added polysaccharides for the food industry in reference to the health claims. Eur. Food Res. Technol. 241(3), 303–315 (2015). https://doi.org/10.1007/s00217-015-2461-7
The Brewers of Europe.: Beer statistics 2017 edition. The Brewers of Europe, Bruxelles (2017)
Ikram, S., Huang, L.Y., Zhang, H.J., Wang, J., Yin, M.: Composition and nutrient value proposition of brewers spent grain. J. Food Sci. 82(10), 2232–2242 (2017). https://doi.org/10.1111/1750-3841.13794
McCarthy, A.L., O’Callaghan, Y.C., Piggott, C.O., FitzGerald, R.J., O’Brien, N.M.: Brewers’ spent grain; bioactivity of phenolic component, its role in animal nutrition and potential for incorporation in functional foods: a review. Proc. Nutr. Soc. 72(1), 117–125 (2013). https://doi.org/10.1017/S0029665112002820
Ozturk, S., Ozboy, O., Cavidoglu, I., Koksel, H.: Effects of brewer’s spent grain on the quality and dietary fibre content of cookies. J. Inst. Brew. 108(1), 23–27 (2002)
Weger, A., Jung, R., Stenzel, F., Hornung, A.: Optimized energetic usage of brewers’ spent grains. Chem. Eng. Technol. 40(2), 306–312 (2017). https://doi.org/10.1002/ceat.201600186
Russ, W., Mörtel, H., Meyer-Pittroff, R.: Application of spent grains to increase porosity in bricks. Constr. Build. Mater. 19(2), 117–126 (2005). https://doi.org/10.1016/j.conbuildmat.2004.05.014
Mishra, P.K., Gregor, T., Wimmer, R.: Utilising brewer’s spent grain as a source of cellulose nanofibres following separation of protein-based biomass. Bioresources. 12(1), 107–116 (2017). https://doi.org/10.15376/biores.12.1.107-116
Chiang, P.C., Chang, P., You, J.H.: Innovative technology fr controlling voc emissions. J. Hazard. Mater. 31(1), 19–28 (1992). https://doi.org/10.1016/0304-3894(92)87036-f
Xiros, C., Christakopoulos, P.: Biotechnological potential of brewers spent grain and its recent applications. Waste Biomass Valoriz. 3(2), 213–232 (2012). https://doi.org/10.1007/s12649-012-9108-8
Carvalheiro, F., Esteves, M.P., Parajó, J.C., Pereira, H., Gírio, F.M.: Production of oligosaccharides by autohydrolysis of brewery’s spent grain. Bioresour. Technol. 91(1), 93–100 (2004). https://doi.org/10.1016/S0960-8524(03)00148-2
Almeida, A.D., Geraldo, M.R.F., Ribeiro, L.F., Silva, M.V., Maciel, M., Haminiuk, C.W.I.: Bioactive compounds from brewer’s spent grain: phenolic compounds, fatty acids and in vitro antioxidant capacity. Acta Sci.-Technol. 39(3), 269–277 (2017). https://doi.org/10.4025/actascitechnol.v39i3.28435
Connolly, A., O’Keeffe, M.B., Piggott, C.O., Nongonierma, A.B., FitzGerald, R.J.: Generation and identification of angiotensin converting enzyme (ACE) inhibitory peptides from a brewers’ spent grain protein isolate. Food Chem. 176, 64–71 (2015). https://doi.org/10.1016/j.foodchem.2014.12.027
Vieira, E., Teixeira, J., Ferreira, I.: Valorization of brewers’ spent grain and spent yeast through protein hydrolysates with antioxidant properties. Eur. Food Res. Technol. 242(11), 1975–1984 (2016). https://doi.org/10.1007/s00217-016-2696-y
Radosavljevic, M., Pejin, J., Kocic-Tanackov, S., Mladenovic, D., Djukic-Vukovic, A., Mojovic, L.: Brewers’ spent grain and thin stillage as raw materials in l-(+)-lactic acid fermentation. J. Inst. Brew. 124(1), 23–30 (2018). https://doi.org/10.1002/jib.462
Gregori, A., Švagelj, M., Pahor, B., Berovič, M., Pohleven, F.: The use of spent brewery grains for Pleurotus ostreatus cultivation and enzyme production. N Biotechnol. 25(2), 157–161 (2008). https://doi.org/10.1016/j.nbt.2008.08.003
Sandhya, C., Sumantha, A., Szakacs, G., Pandey, A.: Comparative evaluation of neutral protease production by Aspergillus oryzae in submerged and solid-state fermentation. Process Biochem. 40(8), 2689–2694 (2005). https://doi.org/10.1016/j.procbio.2004.12.001
Nigam, P.S., Pandey, A.: Biotechnology for agro-industrial residues utilization. Springer, Dordrecht (2009)
Kupski, L., Cipolatti, E., da Rocha, M., Oliveira, M.D., Souza-Soares, L.D., Badiale-Furlong, E.: Solid-state fermentation for the enrichment and extraction of proteins and antioxidant compounds in rice bran by Rhizopus oryzae. Brazil. Arch. Biol. Technol. 55(6), 937–942 (2012). https://doi.org/10.1590/S1516-89132012000600018
Lizardi-Jimenez, M.A., Hernandez-Martinez, R.: Solid state fermentation (SSF): diversity of applications to valorize waste and biomass. 3 Biotech. 7(1), 44 (2017). https://doi.org/10.1007/s13205-017-0692-y
Abd Razak, D.L., Abd Rashid, N.Y., Jamaluddin, A., Sharifudin, S.A., Abd Kahar, A., Long, K.: Cosmeceutical potentials and bioactive compounds of rice bran fermented with single and mix culture of Aspergillus oryzae and Rhizopus oryzae. J. Saudi Soc. Agric. Sci. 16(2), 127–134 (2017). https://doi.org/10.1016/j.jssas.2015.04.001
Cooray, S.T., Chen, W.N.: Valorization of brewer’s spent grain using fungi solid-state fermentation to enhance nutritional value. J. Funct. Foods. 42, 85–94 (2018). https://doi.org/10.1016/j.jff.2017.12.027
Ghosh, B., Ray, R.R.: Current commercial perspective of Rhizopus oryzae: a review. J. Appl. Sci. 11(14), 2470–2486 (2011). https://doi.org/10.3923/jas.2011.2470.2486
Meussen, B.J., de Graaff, L.H., Sanders, J.P., Weusthuis, R.A.: Metabolic engineering of Rhizopus oryzae for the production of platform chemicals. Appl. Microbiol. Biotechnol. 94(4), 875–886 (2012). https://doi.org/10.1007/s00253-012-4033-0
Cantabrana, I., Perise, R., Hernández, I.: Uses of Rhizopus oryzae in the kitchen. Int. J. Gastron. Food Sci. 2(2), 103–111 (2015). https://doi.org/10.1016/j.ijgfs.2015.01.001
Villas-Boas, S.G., Esposito, E., Mitchell, D.A.: Microbial conversion of lignocellulosic residues for production of animal feeds. Anim. Feed Sci. Technol. 98(1–2), 1–12 (2002). https://doi.org/10.1016/s0377-8401(02)00017-2
Lopez, E., Deive, F.J., Longo, M.A., Sanroman, M.A.: Strategies for utilisation of food-processing wastes to produce lipases in solid-state cultures of Rhizopus oryzae. Bioprocess. Biosyst. Eng. 33(8), 929–935 (2010). https://doi.org/10.1007/s00449-010-0416-8
Hsiao, N.-W., Chen, Y., Kuan, Y.-C., Lee, Y.-C., Lee, S.-K., Chan, H.-H., Kao, C.-H.: Purification and characterization of an aspartic protease from the Rhizopus oryzae protease extract. Peptidase R. Electron. J. Biotechnol. 17(2), 89–94 (2014). https://doi.org/10.1016/j.ejbt.2014.02.002
Ibarruri, J., Hernández, I.: Rhizopus oryzae as fermentation agent in food derived sub-products. Waste Biomass Valoriz. 9(11), 2107–2115 (2018). https://doi.org/10.1007/s12649-017-0017-8
Ferreira, J.A., Lennartsson, P.R., Niklasson, C., Lundin, M., Edebo, L., Taherzadeh, M.J.: Spent sulphite liquor for cultivation of an edible Rhizopus sp. Bioresources 7(1), 173–188 (2012)
FazeliNejad, S., Ferreira, J.A., Brandberg, T., Lennartsson, P.R., Taherzadeh, M.J.: Fungal protein and ethanol from lignocelluloses using Rhizopus pellets under simultaneous saccharification, filtration and fermentation (SSFF). Biofuel Res. J. 3(1), 372–378 (2016). https://doi.org/10.18331/brj2016.3.1.7
Canedo, M.S., de Paula, F.G., da Silva, F.A., Vendruscolo, F.: Protein enrichment of brewery spent grain from Rhizopus oligosporus by solid-state fermentation. Bioprocess Biosyst. Eng. 39(7), 1105–1113 (2016). https://doi.org/10.1007/s00449-016-1587-8
Centro de Investigación y Control de la Calidad: Análisis de alimentos: métodos oficiales y recomendados por el Centro de Investigación y Control de la Calidad. Ministerio de Sanidad y Consumo, Madrid (1985)
UNE EN ISO.: Animal feeding stuffs—determination of amylase-treated neutral detergent fibre content (aNDF). (2006)
Satari, B., Karimi, K., Taherzadeh, M.J., Zamani, A.: Co-production of fungal biomass derived constituents and ethanol from citrus wastes free sugars without auxiliary nutrients in airlift bioreactor. Int. J. Mol. Sci. 17(3), 302 (2016). https://doi.org/10.3390/ijms17030302
Bligh, E.G., Dyer, W.J.: A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37(8), 911–917 (1959). https://doi.org/10.1139/o59-099
Waghmare, A.G., Salve, M.K., LeBlanc, J.G., Arya, S.S.: Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour. Bioprocess. 3(1), 1 (2016). https://doi.org/10.1186/s40643-016-0094-8
FAO/WHO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition, vol. 935. WHO Technical Report Series, Geneva (2007)
Nielsen, P.M., Petersen, D., Dambmann, C.: Improved method for determining food protein degree of hydrolysis. J. Food Sci. 66(5), 642–646 (2001). https://doi.org/10.1111/j.1365-2621.2001.tb04614.x
Bougherra, F., Dilmi-Bouras, A., Balti, R., Przybylski, R., Adoui, F., Elhameur, H., Chevalier, M., Flahaut, C., Dhulster, P., Naima, N.: Antibacterial activity of new peptide from bovine casein hydrolyzed by a serine metalloprotease of Lactococcus lactis sub lattice BR16. J. Funct. Foods. 32(Supplement C), 112–122 (2017). https://doi.org/10.1016/j.jff.2017.02.026
Brand-Williams, W., Cuvelier, M.E., Berset, C.: Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 28(1), 25–30 (1995). https://doi.org/10.1016/S0023-6438(95)80008-5
Singleton, V.L., Rossi, J.A.: Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16(3), 144 (1965)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3), 426–428 (1959). https://doi.org/10.1021/ac60147a030
Oliveira, M.d.S., Feddern, V., Kupski, L., Cipolatti, E.P., Badiale-Furlong, E., de Souza-Soares, L.A.: Physico-chemical characterization of fermented rice bran biomass. Caracterización fisico-química de la biomasa del salvado de arroz fermentado. CyTA—J. Food. 8(3), 229–236 (2010). https://doi.org/10.1080/19476330903450274
Rajesh, N., Imelda, J., Raj, R.P.: Value addition of vegetable wastes by solid-state fermentation using Aspergillus niger for use in aquafeed industry. Waste Manag. 30(11), 2223–2227 (2010). https://doi.org/10.1016/j.wasman.2009.12.017
Buenrostro-Figueroa, J.J., Velázquez, M., Flores-Ortega, O., Ascacio-Valdés, J.A., Huerta-Ochoa, S., Aguilar, C.N., Prado-Barragán, L.A.: Solid state fermentation of fig (Ficus carica L.) by-products using fungi to obtain phenolic compounds with antioxidant activity and qualitative evaluation of phenolics obtained. Process Biochem. 62, 16–23 (2017). https://doi.org/10.1016/j.procbio.2017.07.016
Ajila, C.M., Gassara, F., Brar, S.K., Verma, M., Tyagi, R.D., Valéro, J.R.: Polyphenolic antioxidant mobilization in apple pomace by different methods of solid-state fermentation and evaluation of its antioxidant activity. Food Bioprocess Technol. 5(7), 2697–2707 (2012). https://doi.org/10.1007/s11947-011-0582-y
Fruet, A.P.B., Stefanello, F.S., Rosado Júnior, A.G., Souza, A.N.M.d., Tonetto, C.J., Nörnberg, J.L.: Whole grains in the finishing of culled ewes in pasture or feedlot: performance, carcass characteristics and meat quality. Meat Sci. 113, 97–103 (2016). https://doi.org/10.1016/j.meatsci.2015.11.018
Paraskevakis, N.: Effects of dietary dried Greek Oregano (Origanum vulgare ssp. hirtum) supplementation on blood and milk enzymatic antioxidant indices, on milk total antioxidant capacity and on productivity in goats. Anim. Feed Sci. Technol. 209, 90–97 (2015). https://doi.org/10.1016/j.anifeedsci.2015.09.001
Castillo, C., Pereira, V., Abuelo, A., Hernandez, J.: Effect of supplementation with antioxidants on the quality of bovine milk and meat production. Sci. World J. (2013) https://doi.org/10.1155/2013/616098
Wang, D., Sakoda, A., Suzuki, M.: Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain. Bioresour. Technol. 78(3), 293–300 (2001). https://doi.org/10.1016/S0960-8524(01)00002-5
Dulf, F.V., Vodnar, D.C., Socaciu, C.: Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 209, 27–36 (2016). https://doi.org/10.1016/j.foodchem.2016.04.016
Correia, R.T.P., McCue, P., Magalhães, M.M.A., Macêdo, G.R., Shetty, K.: Production of phenolic antioxidants by the solid-state bioconversion of pineapple waste mixed with soy flour using Rhizopus oligosporus. Process Biochem. 39(12), 2167–2172 (2004). https://doi.org/10.1016/j.procbio.2003.11.034
Oliveira, M.D., Feddern, V., Kupski, L., Cipolatti, E.P., Badiale-Furlong, E., de Souza-Soares, L.A.: Changes in lipid, fatty acids and phospholipids composition of whole rice bran after solid-state fungal fermentation. Bioresour. Technol. 102(17), 8335–8338 (2011). https://doi.org/10.1016/j.biortech.2011.06.025
FEDNA. Fibra neutro detergente, Ácido detergente Y Lignina (FND,FAD,LAD secuenciales). http://fundacionfedna.org/tecnicas_de_analisis/fibra-neutro-detergente-%C3%A1cido-detergente-y-lignina-fndfadlad-secuenciales. Accessed 30 Sep, 2018
Ferret, A., Calsamiglia, S., Bach, A., Devant, M., Fernández, C., García-Rebollar, P.: Necesidades nutricionales para rumiantes de cebo. In: FEDNA (Fundación Española para el Desarrollo de la Nutrición Animal) (2008)
Kaur, V.: Incorporation of brewery waste in supplementary feed and its impact on growth in some carps. Bioresour. Technol. 91(1), 101–104 (2004). https://doi.org/10.1016/s0960-8524(03)00073-7
Miles, R.D., Chapman, F.A.: The benefits of fish meal in aquaculture diets. Institute of Food and Agricultural Sciences, University of Florida, Florida (2006)
Asadollahzadeh, M., Ghasemian, A., Saraeian, A., Resalati, H., Taherzadeh, M.: Production of fungal biomass protein by filamentous fungi cultivation on liquid waste streams from pulping process. BioResources. 13(1), 5013–5031 (2018). https://doi.org/10.15376/biores.13.3.5013-5031
Nitayavardhana, S., Issarapayup, K., Pavasant, P., Khanal, S.K.: Production of protein-rich fungal biomass in an airlift bioreactor using vinasse as substrate. Bioresour. Technol. 133, 301–306 (2013). https://doi.org/10.1016/j.biortech.2013.01.073
Wei, D., Li, M., Zhang, X., Ren, Y., Xing, L.: Identification and characterization of a novel delta12-fatty acid desaturase gene from Rhizopus arrhizus. FEBS Lett. 573(1–3), 45–50 (2004). https://doi.org/10.1016/j.febslet.2004.06.100
Innes, J.K., Calder, P.C.: Prostaglandins: Omega-6 fatty acids and inflammation. Leukot. Essent. Fatty Acids. 132, 41–48 (2018). https://doi.org/10.1016/j.plefa.2018.03.004
Acknowledgements
Authors thank to Boga Cooperative for providing the BSG. This work was funded by the Basque Government (Department of Economic and Infrastructure Development, Agriculture, Fisheries and Food policy). This paper is Contribution No. 901 from AZTI (Food Research).
Funding
Funding was provided by Ekonomiaren Garapen eta Lehiakortasun Saila, Eusko Jaurlaritza.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ibarruri, J., Cebrián, M. & Hernández, I. Solid State Fermentation of Brewer’s Spent Grain Using Rhizopus sp. to Enhance Nutritional Value. Waste Biomass Valor 10, 3687–3700 (2019). https://doi.org/10.1007/s12649-019-00654-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00654-5