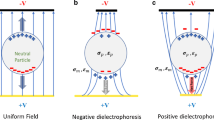
Abstract
Separation of biological cells from the mixture has been studied over a period of time in medicine and basic environmental research. Dielectrophoresis (DEP) is one among the portable techniques to separate the cells with minimum requirements such as electric field and effective medium. With the advent of BioMEMS, the understanding of dielectric characteristics of various biological cells has become imperative. Dielectric properties of the biological cells are studied by the extensive involvement of bioelectrokinetics with respect to the electric field. This has led to the accumulation of data on properties of cells, particularly with respect to dielectrophoresis (DEP). This article reviews the bioelectrokinetics of biological cells and how they are exploited in cell separation using DEP.



Similar content being viewed by others
References
Yoon, Y.K., Park JH, Cros, F., Allen, M.G. (2003) Integrated vertical screen microfilter system using inclined SU-8 structures, in: Proceedings of the 16th International Conference on Micro Electro Mechanical Systems 2003 (MEMS’03), Kyoto, Japan, 227– 230.
Fu, A. Y., Spence, C., Scherer, A., Arnold, F. H., Quake, S. R. (1999). A microfabricated fluorescence-activated cell sorter. Nature Biotechnology, 17, 1109–1111.
Chalmers, J. J., Zborowski, M., Sun, L., Moore, L. (1998). Flow through Immunomagnetic cell separation. Biotechnology Progress, 14, 141.
Cheng, I.-F., Froude, V., Zhu, Y. E., Chang, H.-C., Chang, H.-C. (2009). A continuous high-throughput bioparticle sorter based on 3D traveling-wave dielectrophoresis. Lab on a Chip, 9, 3193–3201.
Zhang, L., Tatar, F., Turmezei, P., Bastemeijer, J., Mollinger, J. R., Piciu, O., et al. (2006). Continuous electrodeless dielectrophoretic separation in circular channel. Journal of Physics, 34, 527–532.
Doh, I., & Cho, Y. H. (2005). A continuous cell separation chip using hydrodynamic dielectrophoresis (DEP) process. Sensors and Actuators A, 121, 59–65.
Armstrong, D. W., Schulte, G., Schneiderheinze, J. M., Westenberg, D. J. (1999). Separating microbes in the manner of molecules. Capillary electrokinetic approaches. Analytical Chemistry, 71, 5465–5469.
Jones, T. B. (1995). Electromechanics of particles. Cambridge, UK: Cambridge University Press.
Pohl, H. (1978). Dielectrophoresis. Cambridge: Cambridge University Press.
Burt, J. P. H., Pethig, R., Gascoyne, P. R. C., Becker, F. F. (1990). Dielectrophoretic characterisation of friend murine erythroleukaemic cells as a measure of induced differentiation. Biochimica et Biophysica Acta, 1034(1), 93–101.
Kovacic, P., & Pozos, R. S. (2007). Bioelectronome. Integrated approach to receptor chemistry, radicals, electrochemistry, cell signaling, and physiological effects based on electron transfer. Journal of Receptor and Signal Transduction Research, 27(4), 261–94.
Kovacic, P., Pozos, R. S., Draskovich, C. D. (2007). Unifying electrostatic mechanism for receptor-ligand activity. Journal of Receptor and Signal Transduction Research, 27(5–6), 411–31.
Zhang, C., Khoshmanesh, K., Mitchell, A., Kalantarzadeh, K. (2010). Dielectrophoresis for manipulation of micro/nano particles in microfluidic systems. Analytical and Bioanalytical Chemistry, 396, 401–420.
Alshareef, M., Metrakos, N., Perez, E. J., Azer, F., Yang, F., et al. (2013). Separation of tumor cells with dielectrophoresis-based microfluidic chip. Biomicrofluidics, 7, 01180. doi:10.1063/1.4774312.
Patel, S., Showers, D., Vedantam, P., Tzeng, T. R., Qian, S. (2012). Xuan X (2012) Microfluidic separation of live and dead yeast cells using reservoir-based dielectrophoresis. Biomicrofluidics, 6, 034102.
Gonzalez, C. F., & Remcho, V. T. (2005). Harnessing dielectric forces for separations of cells, fine particles and macromolecules. Journal of Chromatography. A, 1079, 59–68.
Lei Pei-Hou Sun, U., & Pethig, R. (2011). Refinement of the theory for extracting cell dielectric properties from dielectrophoresis and electrorotation experiments. Biomicrofluidics, 5, 044109.
Fricke, H. (1955). The complex conductivity of a suspension of stratified particles of spherical or cylindrical form. Journal of Physical Chemistry, 59, 168–170.
Fricke, H. (1953). The electric permittivity of a dilute suspension of membrane-covered ellipsoids. Journal of Applied Physics, 24, 644–6.
Chen, J., Abdelgawad, M., Yu, L., Shakiba, N., Chien, W. Y., Lu, Z., et al. (2011). Electrodeformation for single cell mechanical characterization. Journal of Micromechanics and Microengineering, 21, 054012.
Kregiel, D., Berlowoska, J., Szubzda, B. (2012). Novel permittivity test for determination of yeast surface charge and flocculation abilities. Journal of Industrial Microbiology and Biotechnology. doi:10.1007/s10295 – 012- 1193 – y.
Pauly, H., Packer, L., Schwan, H. P. (1960). Electrical properties of mitochondrial membranes. Journal of Cell Biology, 7(4), 589–601.
Maxwell, J. C. (1873). A treatise on electricity and magnetism Vol 1. Oxford U K: Clarendon.
Wagner, K. W. (1914). The after effects in di electrics. Archiv Elektrotechnik, 2, 371–87.
Carstensen, E. L., Cox, H. A., Mercer, W. B., Natale, L. A. (1965). Passive electrical properties of microorganisms: Conductivity of Escherichia coli and Micrococcus lysodeikticus. Biophysical Journal, 5(3), 289–300.
Cevc, G. (1990). Membrane electrostatics. Biochimica et Biophysica Acta, 1031, 311–82.
Pethig, R. (1979). Dielectric and electronic properties of biological systems: Their significance in physiology, biophysics and biotechnology. Physics in Medicine and Biology, 32, 933–70.
Sonohara, R., Muramatsu, N., Ohshima, H., Kondo, T. (1995). Difference in surface properties between Escherichia coli and Staphylococcus aureus as revealed by electrophoretic mobility measurements. Biophysical Chemistry, 55, 273–277.
Markx, G. H., Pethig, R., Rousselet, J. (1997). The dielectrophoretic levitation of latex beads, with reference to field-flow fractionation. Journal of Physics D: Applied Physics, 30, 2470–2477.
Gurtovenko, A. A., & Vattulainen, I. (2008). Membrane potential and electrostatics of phospholipid bilayers with asymmetric transmembrane distribution of anionic lipids. Journal of Physical Chemistry B, 112, 4629–4634.
Andreev, V. P. (2013). Cytoplasmic electric fields and electroosmosis: Possible solution for the paradoxes of the intracellular transport of biomolecules. PLoS ONE, 8(4), e61884. doi:10.1371/journal.pone.0061884.
Singh, A., Orsat, V., Raghavan, V. (2013). Soybean hydrophobic protein response to external electric field: a molecular modeling approach. Biomolecules, 3, 168–179.
Castellarnau, M., Errachid, A., Madrid, C., Juarez, A., Samitier, J. (2006). Dielectrophoresis as a tool to characterize and differentiate isogenic mutants of Escherichia coli. Biophysical Journal, 91(2006), 3937–3945.
Gatenby, R. A., & Frieden, B. R. (2010). Coulomb interactions between cytoplasmic electric fields and phosphorylated messenger proteins optimize information flow in cells. PLoS One, 5(8), e12084.
Pethig, R., & Markx, G. H. (1997). Applications of dielectrophoresis in biotechnology. Trends in Biotechnology, 15, 426–432.
Davey, C.L., Kell, D. B. (1995) The low frequency dielectric properties of biological cells. In. Waltz D, Berg H, Milazzo G, editors. Bioelectrochemistry of cells and tissues. Zurich: Birkauser.159 – 207.
Regtmeier, J., Duong, T. T., Eichhorn, R., Anselmetti, D., Ros, A. (2007). Dielectrophoretic manipulation of DNA: Separation and polarizability. Analytical Chemistry, 79, 3925–3932.
Morgan, H., & Green, G. (2003). Electrokinetic technologies for sub-micron particles. Philadelphia: Research Studies Press.
Holzel, R., Calander, N., Chiragwandi, Z., Willander, M., Bier, F. F. (2005). Trapping single molecules by Dielectrophoresis. Physical Review Letters, 95, 128102–4.
Porschke, D. (1997). Macrodipoles: Unusual electric properties of biological macromolecules. Biophysical Chemistry, 66, 241–257.
Chou, C. F., Tegenfeldt, J. O., Bakajin, O., Chan, S. S., Cox, E. C., Darton, N., et al. (2002). Electrodeless dielectrophoresis of single- and double-stranded DNA. Biophysical Journal, 83, 2170–2179.
Henning, A., Bier, F. F. F., Holzel, R. (2010). Dielelctophoresis of DNA: Quantification by impedance measurements. Biomicrofluidics, 4, 022803.
Regtmeier, J., Eichhorn, R., Bogunovic, L., Ros, A., Anselmetti, D. (2010). Dielectrophoretic trapping and polarizability of DNA: the role of spatial conformation. Analytical Chemistry, 82, 7141–7149.
Markx, G. H., Huang, Y., Zhou, X. F., Pethig, R. (1994). Dielectrophoretic characterisation and separation of micro-organisms. Microbiology UK, 140, 585–591.
Morgan, H., Ginzburg, M., Ginzburg, B. Z. (1987). Dielectric properties of the halophilic bacteria Halobacterium halobium and H. marismortui with reference to the conductivities and permittivities of the cytoplasmic membrane and intracellular phases. Biochimica et Biophysica Acta, 924(1), 54–66.
Abo - Shady, A. M., Mohamed, Y. A., Lasheen, T. (1993). Chemical composition of the cell wall in some green algae species. Biologia Plantarum, 35(4), 629–632.
Hardison, S. E., & Brown, G. D. (2012). Structure of the fungal cell wall. Nature Immunology, 13, 817–822.
Sendbuschand, P. V. (2003). Cell walls of Algae. Botany Online, 7(31), 10–29. Retrived on 2007.
Khoshmanesh, K., Baratchi, S., Tovar-Lopez, F. J., Nahavandi, S., Wlodkowic, D., Mitchell, A., et al. (2012). On-chip separation of Lactobacillus bacteria from yeasts using dielectrophoresis. Microfluidics and Nanofluidics, 12, 597–606.
Moncada-Hernández, H., & Lapizco-Encinas, B. H. (2010). Simultaneous concentration and separation of microorganisms: Insulator-based dielectrophoretic approach. Analytical and Bioanalytical Chemistry, 396, 805–1816.
Denga, Y. L., Chang, J, S, Juang,Y. J., (2012) Separation of microalgae with different lipid contents by dielectrophoresis. Bioresource Technology.
Gallo Villanueva, R. C., Perez, N. M., Martnez-Lopez, J. I., Lapizco-Encinas, P. A. (2011). Assessment of microalgae viability employing insulator-based dielectrophoresis. Microfluidics and Nanofluidics, 10, 1305–1315.
Lee, K. J. D., Marcus, S. E., Knux, J. P. (2011). Cell wall biology: Perspectives from cell wall imaging. Molecular Plant, 4, 212–219.
Jutiporn, S., Wanichapichart, P., Mahaworasilpa, T., Coster, H. G. L. (1999). AC electric field frequencies for isolating five marine phytoplanktons. Songklanakarin J Sci Technol, 21(2), 213–9.
Nieper, H. A. (1985). Dr. Nieper’s Revolution in Technology, Medicine and Societ. Oldenburg: MIT Verlag.
Hughes, M. P., Wang, X., Burt, J. P. H, Pethig, R., Watkins, L. R.., (1994) In: Proceedings of 2nd International Conference on Comp. Electromag 38: 48–51.
Yang, J., Huang, Y., Wang, X., Becker, F. F., Gascoyne, P. R. C. (1999). Dielectric Properties of human leukocyte subpopulations determined by electrorotation as a cell separation criterion. Biophysical Journal, 76, 3307–3314.
Yang, J., Huang, Y., Wang, X., Becker, F. F., Gascoyne, P. R. C. (2000). Differential analysis of human leukocytes by dielectrophoretic field-flow-fractionation. Biophysical Journal, 78, 2680–2689.
Chan, K. L., Morgan, H., Morgan, E., Cameron, I. T., Thomas, M. R. (2000). Measurements of the dielectric properties of peripheral blood mononuclear cells and trophoblast cells using AC electrokinetic technique. Biochimica et Biophysica Acta, 1500, 313–322.
Muratore, M., Srsen, V., Waterfall, M., Downes, A., Pethig, R. (2012). Biomarker-free dielectrophoretic sorting of differentiating myoblast multipotent progenitor cells and their membrane analysis by Raman spectroscopy. Biomicrofluidics, 6, 034113.
Kucera, O., & Havelka, D. (2012). Mechano-electrical vibrations of microtubules—Link to subcellular morphology. BioSystems, 109, 346–355.
Mazzanti, M., Bustamante, J. O., Oberleithner, H. (2001). Electrical dimension of the nuclear envelope. Physiological Reviews, 81, 1–19.
Becker, F. F., Wang, X. B., Huang, Y., Pethig, R., Vykoukal, J., Gascoyne, P. R. (1995). Separation of human breast cancer cells from blood by differential dielectric affinity. Proceedings of the National Academy of Science USA Cell Biology, 92, 860–864.
Blad, B., & Baldetorp, B. (1996). Impedance spectra of tumour tissue in comparison with normal issue: a possible clinical application for electrical impedance tomography. Physiological Measurement, 17, 105–115.
Cone, C. D. (1985). Transmembrane potentials and characteristics of immune and tumor cells. Boca Raton Florida: CRC Press.
Gupta, V., Jafferji, I., Garza, M., Melnikova, V. O., Hasegawa, D. K., et al. (2012). ApoStream, a new dielectrophoretic device for antibody independent isolation and recovery of viable cancer cells from blood. Biomicrofluidics, 6, 024133.
Salmanzadeh, A., Kittur, H., Sano, M. B., Roberts, P. C., Schmelz, E. M., Davalos, R. V. (2012). Dielectrophoretic differentiation of mouse ovarian surface epithelial cells, macrophages, and fibroblasts using contactless dielectrophoresis. Biomicrofluidics, 6, 024104.
Cima, I., Yee, C. W., Iliescu, F. S., Phyo, W. M., Lim, K. H., Iliescu, C., et al. (2013). Label-free isolation of circulating tumor cells in microfluidic devices: Current research and perspectives. Biomicrofluidics, 7, 011810.
Nikolic-Jaric, M., Cabel, T., Salimi, E., Bhide, A., Braasch, K., et al. (2013). Differential electronic detector to monitor apoptosis using dielectrophoresis induced translation of flowing cells (dielectrophoresis cytometry). Biomicrofluidics, 7, 024101.
Lapizco-Encinas, B. H., Simmons, B. A., Cummings, E. B. (2004). Insulator-based dielectrophoresis for the selective concentration and separation of live bacteria in water. Electrophoresis, 25, 1695–1704.
Li, H., & Bashir, R. (2002). Dielectrophoretic separation and manipulation of live and heat-treated cells of Listeria on microfabricated devices with interdigitated electrodes. Sensors and Actuator B. Chemical, 86, 215–221.
Jen, C. –. P., & Chen, T. –. w. (2009). Selective trapping of live and dead mammalian cells using insulator based dielectrophoresis within open–top microstructures. Biomedical Microdevices, 11, 597–607.
Jen, C. P., & Chen, T. W. (2009). Selective trapping of live and dead mammalian cells using insulator-based dielectrophoresis within open-top microstructures. Biomedical Microdevices, 11(3), 597–607.
Huang, Y., Holzel, R., Pethig, R., Wang, X. B. (1992). Differences in the AC electrodynamics of viable and non-viable yeast cells determined through combined dielectrophoresis and electrorotation studies. Physics in Medicine and Biology, 37(7), 499–1517.
Docoslis, A., Espinoza, L. A. T., Zhang, B., Cheng, L. L., Israel, B. A., Alexandridis, P., et al. (2007). Using non uniform electric fields to accelerate the transport of viruses to surfaces from media of physiological ionic strength. Langmuir, 23, 3840–3848.
Moon, H. S., Nam, Y. W., Park, J. C., Jung, H. I. (2009). Dielectrophoretic separation of airborne microbes and dust particles using a microfluidic channel for real-time bioaerosol monitoring. Environmental Science and Technology, 43(15), 5857–5863.
Hughes, M. P., Hotteges, K. F., (2008) Bacterial Concentration, Separation and Analysis by Dielectrophoresis. Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems 895-907.
Li, S., Li, M., Robin, K. B., Cao, W., Yeung, I., Chau, Y., et al. (2013). High-throughput particle manipulation by hydrodynamic, electrokinetic, and dielectrophoretic effects in an integrated microfluidic chip. Biomicrofluidics, 7, 024106.
Velev, O. D., & Bhatt, K. H. (2006). On-chip micromanipulation and assembly of colloidal particles by electric fields. The Royal Society of Chemistry, Soft Matter, 2, 738–750.
Gordon, J. E., Gagnon, Z., Chang, H. C. (2007). Dielectrophoretic discrimination of bovine red blood cell starvation age by buffer selection and membrane cross linking. Biomicrofluidics, 1, 044102.
Suscillon, C., Velev, O. D., Slaveykova, V. I. (2013). Alternating current-dielectrophoresis driven on-chip collection and chaining of green microalgae in freshwaters. Biomicrofluidics, 7, 024109.
Wu, L., Yung, L. Y. L., Lim, K. M. (2012). Dielectrophoretic capture voltage spectrum for measurement of dielectric properties and separation of cancer cells. Biomicrofluidics, 6, 014113.
Knox, J. H., & McCormack, K. A. (1994). Temperature effects in capillary electrophoresis 2: Some theoretical calculations and predictions. Chromatographia, 38, 215–221.
Rush, R. S., Cohen, A. S., Cohen, A. S., Karger, B. L. (1991). Influence of column temperature on the electrophoretic behavior of myoglobin and alpha-lactalbumin in high-performance capillary electrophoresis. Analytical Chemistry, 63, 1346–1350.
Castellanos, A., Ramos, A., Gonzalez, A., Green, N. G., Morgan, H. (2003). Electrohydrodynamics and dielectrophoresis in microsystems: Scaling law. Journal of Physics D: Applied Physics, 36, 2584–2597.
Jones, P. V., Staton, S. J. R., Hayes, M. A. (2011). Blood cell capture in a sawtooth dielectrophoretic microchannel. Analytical and Bioanalytical Chemistry, 401, 103–2111.
Gupta, S., (2007) On-chip Assembly of Electrically Functional Structures from Biological and Colloidal Particles. Dissertation, North Carolina State University.
Noble, P. A., Dziuba, M., Harrison, D. J., Albritton, W. L. (1999). Factors influencing capacitance-based monitoring of microbial growth. Journal of Microbiological Methods, 37, 51–64.
Beveridge, T.J., (1988) Wall ultrastructure: how little we know. InActor, P., Daneo-Moore, L., Higgins, M.L., Salton, M.R.J., Shockman, G.D. (Eds.), Antibiotic Inhibition of Bacterial cell Surface Assembly and Function. American Society for Micro- biology, Washington, DC, USA, 3–20.
Beveridge, T. J., & Graham, L. L. (1991). Surface layers of bacteria. Microbiological Reviews, 55, 684–705.
Champlin, F. R., Patterson, C. E., Austin, F. W., Ryals, P. E. (1999). Derivation of extracellular polysaccharide-deficient variants from a serotype A strain of Pasteurella multocida. Current Microbiology, 38, 268–272.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Devi, U.V., Puri, P., Sharma, N.N. et al. Electrokinetics of Cells in Dielectrophoretic Separation: A Biological Perspective. BioNanoSci. 4, 276–287 (2014). https://doi.org/10.1007/s12668-014-0140-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-014-0140-y