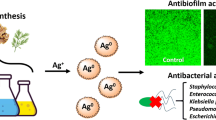
Abstract
Green chemistry is generating an increasing amount of interest for the synthesis of metal nanoparticles due to the cost-effectiveness and eco-friendly nature of the plants. The green approach of nanoparticle synthesis is a better substitute for the chemical and physical methods since there is no involvement of toxic chemicals. The objective of the present research was the synthesis of silver nanoparticles (AgNPs) using bark extract of the medicinal plant Holarrhena pubescens Wall ex G. Don (HP) and evaluating their antibacterial and antibiofilm properties against clinical isolates of P. aeruginosa. The HP-AgNPs were characterized by ultraviolet-visible spectroscopy, Fourier transform infra red, atomic force microscopy, and transmission electron microscopy (TEM). The synthesized HP-AgNPs were spherical in shape, and the average particle size as analyzed by TEM was 13.15 nm. The antibacterial potential of HP-AgNPs was evaluated by determining MIC (minimum inhibitory concentration) and MBC (minimum bactericidal concentration). The MIC was found to be in the range of 20–25 μg/ml. Further, the interaction of AgNPs with imipenem-resistant P. aeruginosa was analyzed by TEM. The rupturing of the cell membrane and cell wall was seen at the lethal concentration, and the nanoparticles entered the cell from the several sites that caused great damage to the cell which eventually led to cell death. The antibiofilm properties of HP-AgNPs against imipenem-resistant P. aeruginosa were demonstrated by CLSM (confocal laser scanning microscopy) using propidium iodide and ConA-FITC dye. CLSM images clearly show that as the concentration of HP-AgNPs increased, the number of biofilm-forming cells decreased due to the cell death. It was also observed that AgNPs inhibit or restrict the colonization and halts the formation of biofilm. The result obtained suggested that green-synthesized HP-AgNPs have great antibacterial and antibiofilm potential and could be used as an alternative agent to treat the infection caused by imipenem-resistant P. aeruginosa.







Similar content being viewed by others
References
Ansari, M. A., Khan, H. M., Khan, A. A., Sultan, A., & Azam, A. (2012). Synthesis and characterization of the antibacterial potential of ZnO nanoparticles against extended-spectrum b-lactamases producing Escherichia coli and Klebsiella pneumoniae isolated from a tertiary care hospital of North India. Applied Microbiology and Biotechnology, 94, 467–477.
Bereket, W., Hemalatha, K., Getenet, B., Wondwossen, T., Solomon, A., Zeynudin, A., & Kannan, S. (2012). Update on bacterial nosocomial infections. Euro Rev Med Pharmacol Sci., 16, 1039–1044.
Su, H. C., Ramkissoon, K., Doolittle, J., Clark, M., Khatun, J., Secrest, A., et al. (2010). The development of ciprofloxacin resistance in Pseudomonas aeruginosa involves multiple response stages and multiple proteins. Antimicrobial Agents and Chemotherapy, 54, 4626–4635.
Juan, C., Zamorano, L., Perez, J. L., Ge, Y., Oliver, A., et al. (2010). Activity of a new antipseudomonal cephalosporin, CXA-101 (FR264205), against carbapenem-resistant and multidrug resistant Pseudomonas aeruginosa clinical strains. Antimicrobial Agents and Chemotherapy, 54, 846–851.
DeQueiroz, G. A., & Day, D. F. (2007). Antimicrobial activity and effectiveness of a combination of sodium hypochlorite and hydrogen peroxide in killing and removing Pseudomonas aeruginosa biofilms from surfaces. Journal of Applied Microbiology, 103, 794–802.
Davis, S. C., Martinez, L., & Kirsner, R. (2006). The diabetic foot: the importance of biofilms and wound bed preparation. Curr. Diabet. Rep., 6, 439–445.
Smith, A. W. (2005). Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Advanced Drug Delivery Reviews, 57, 1539–1550.
Pal, S., Tak, Y. K., & Song, J. M. (2007). Dose the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Applied and Environmental Microbiology, 73, 1712–1720.
Weir, E., Lawlor, A., Whelan, A., & Regan, F. (2008). The use of nanoparticles in anti- microbial materials and their characterization. The Analyst, 133, 835–845.
Muhling, M., Bradford, A., Readman, J. W., Somerfield, P. J., & Handy, R. D. (2009). An investigation into the effects of silver nanoparticles on antibiotic resistance of naturally occurring bacteria in an estuarine sediment. Marine Environmental Research, 68, 278–283.
Yeo, S. Y., Lee, H. J., & Jeong, S. H. (2003). Preparation of nanocomposite fibers for permanent antibacterial effect. Journal of Materials Science, 38, 2143–2147.
Lok, C. N., Ho, C. M., Chen, R., He, Q. Y., Yu, W. Y., Sun, H., et al. (2006). Proteomic analysis of the mode of antibacterial action of silver nanoparticles. Journal of Proteome Research, 5, 916–924.
Gogoi, S. K., Gopinath, P., Paul, A., Ramesh, A., Ghosh, S. S., & Chattopadhyay, A. (2006). Green fluorescent protein-expressing Escherichia coli as a model system for investigating the antimicrobial activities of silver nanoparticles. Langmuir, 22, 9322–9328.
Alt, V., Bechert, T., Steinrücke, P., Wagener, M., Seidel, P., Dingeldein, E., et al. (2004). Nanoparticulate silver. A new antimicrobial substance for bone cement. Orthopade, 33, 885–892.
Panacek, A., Kvitek, L., Prucek, R., Kolar, M., Vecerova, R., Pizúrova, N., et al. (2006). Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. The Journal of Physical Chemistry. B, 110, 16248–16253.
Baker, C., Pradhan, A., Pakstis, L., Pochan, D. J., & Shah, S. I. (2005). Synthesis and antibacterial properties of silver nanoparticles. Journal of Nanoscience and Nanotechnology, 5, 244–249.
Morones, J. R., Elechiguerra, J. L., Camacho, A., Holt, K., Kouri, J. B., Ramírez, J. T., et al. (2005). The bactericidal effect of silver nanoparticles. Nanotechnol, 16, 2346–2353.
Ip, M., Lui, S. L., Poon, V. K., Lung, I., & Burd, A. (2006). Antimicrobial activities of silver dressings: an in vitro comparison. Journal of Medical Microbiology, 55, 59–63.
Vilchis-Nestor, A. R., Sanchez-Mendieta, V., Camacho-Lopez, M. A., Gomez-Espinosa, R. M., Camacho-Lopez, M. A., & Arenas-Alatorre, J. A. (2008). Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Materials Letters, 62, 3103–3105.
Shankar, S. S., Rai, A., Ahmad, A., & Sastry, M. (2004). Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. Journal of Colloid and Interface Science, 275, 496–502.
Sastry, M., Ahmad, A., Khan, M. I. and Kumar, R. (2004). Microbial nanoparticle production, in nanobiotechnology: concepts, applications and perspectives (eds C. M. Niemeyer and C. A. Mirkin), Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, FRG. doi: https://doi.org/10.1002/3527602453.ch9.
Bhattacharya, D., & Gupta, R. K. (2005). Nanotechnology and potential of microorganisms. Critical Reviews in Biotechnology, 25, 199–204.
Mohanpuria, P., Rana, N. K., & Yadav, S. K. (2008). Biosynthesis of nanoparticles: technological concepts and future applications. Journal of Nanoparticle Research, 10, 507–517.
Singh, K., Panghal, M., Kadyan, S., Chaudhary, U., & Yadav, J. P. (2014). Antibacterial activity of synthesized silver nanoparticles from Tinospora cordifolia against multi drug resistant strains of Pseudomonas aeruginosa isolated from burn patients. Journal Nanomed Nanotechnol, 5, 192.
Yadav, J. P., Kumar, S., Budhwar, L., Yadav, A., & Yadav, M. (2016). Characterization and antibacterial activity of synthesized silver and iron nanoparticles using Aloe vera. Journal of Nanomedicine & Nanotechnology, 7, 384.
Ali, S. G., Ansari, M. A., Khan, H. M., Jalal, M., Mahdi, A. A., & Cameotra, S. S. (2017). Crataeva nurvala nanoparticles inhibit virulence factors and biofilm formation in clinical isolates of Pseudomonas aeruginosa. Journal Basic Microbiology, 57, 193–203.
Ali, S. G., Khan, H. M., Jalal, M., Ansari, M. A., Mahdi, A. A., & Ahmad, M. K. (2015). Green synthesis of silver nanoparticles using the leaf extract of Putranjiva roxburghii wall. and their antimicrobial activity. Asian journal of Pharmaceutical and Clinical Research, 8, 335–338.
Bhutani, K. K. (1984). Proceedings of national symposium of applied biotechnology of medicinal, aromatic and timber yielding plants. University of Calcutta, India;. pp. 387–392.
Sharma, P.C., Yelen, M.B., and Dennis, T. J. (2004). In database on medicinal plants used in Ayurveda, Vol 2. New Delhi; Central Council for Research in Ayurveda and Siddha; p.550.
Bhattacharjee, S. K. (2004). Handbook of medicinal plants. Jaipur: Pointer Publishers.
Daniel, M. (2006). Medicinal plants. New Delhi: Oxford of IBH Publishing.
Kumar, N., Singh, B., Bhandari, P., Gupta, A. P., & Kaul, V. K. (2007). Steroidal alkaloids from Holarrhena antidysenterica (L.) wall. Chemical and Pharmaceutical Bulletin., 55, 912–914.
Tille, M.P. (2013). Pseudomonas, Burkholderia and similar organism In: Bailey & Scott’s Diagnostic Microbiology, 13 edn. Elsevier, Ch-22, pp 335–347.
Clinical Laboratory Standards Institute (2012). Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. Document M100-S22. Clinical laboratory standards institute, Wayne, PA.
Banas, J. A., Hazlet, K. R. O., & Mazurkiewicz, J. E. (2001). An in vitro model for studying the contributions of the Streptococcus mutans glucan-binding protein—a to biofilm structure. Meth. Enzymol., 337, 425–433.
Zhao, G., & Stevens, S. E. (1998). Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals, 11, 27–32.
Liu, Q., Li, X., Li, W., Du, X., He, J. Q., Tao, C., & Feng, Y. (2015). Influence of carbapenem resistance on mortality of patients with Pseudomonas aeruginosa infection: a meta-analysis. Scientific Reports, 5, 11715.
Buehrle, D. J., Shields, R. K., Clarke, L. G., Potoski, B. A., Clancy, C. J., & Nguyen, M. H. (2017). Carbapenem-resistant Pseudomonas aeruginosa bacteremia: risk factors for mortality and microbiologic treatment failure. Antimicrobial Agents and Chemotherapy, 61, e01243–e01216.
Sondi, I., & Salopek-Sondi, B. (2004). Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram-negative bacteria. Journal of Colloid and Interface Science, 275, 177–182.
Bao, H., Yu, X., Xu, C., Li, X., Li, Z., Wei, D., & Liu, Y. (2015). New toxicity mechanism of silver nanoparticles: promoting apoptosis and inhibiting proliferation. PLoS One, 10(3), e0122535.
Ali, S. G., Ansari, M. A., Jamal, Q. M. S., Khan, H. M., Jalal, M., Ahmad, H., & Mahdi, A. A. (2017). Antiquorum sensing activity of silver nanoparticles in P. aeruginosa: an in silico study. In Silico Pharmacol, 5, 12. https://doi.org/10.1007/s40203-017-0031-3
Singh, K., Panghal, M., Kadyan, S., Chaudhary, U., & Yadav, J. P. (2014). Green silver nanoparticles of Phyllanthus amarus: as an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. Journal of Nanobiotechnology, 12, 40.
Rani, R., Sharma, D., Chaturvedi, M., & Yadav, J. P. (2017). Green synthesis, characterization and antibacterial activity of silver nanoparticles of endophytic fungi Aspergillus terreus. Journal Nanomed Nanotechnol, 8, 457.
Suzuki, T., Fujikura, K., Higashiyam, T., & Takata, K. (1997). DNA staining for fluorescence and laser confocal microscopy. The Journal of Histochemistry and Cytochemistry, 45, 49–53.
Kalishwaralal, K., Barath Mani Kanth, S., Pandian, S. R. K., Deepak, V., & Gurunathan, S. (2010). Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids and Surfaces. B, Biointerfaces, 79, 340–344.
Ansari, M. A., Khan, H. M., Khan, A. A., Cameotra, S. S., & Pal, R. (2013). Antibiofilm efficacy of silver nanoparticles against biofilm of extended spectrum b-lactamase isolates of Escherichia coli and Klebsiella pneumoniae. Applied Nanoscience, 4, 859–868.
Acknowledgments
The authors would like to acknowledge AIRF, Jawaharlal Nehru University, New Delhi, India, for the CLSM and to All India Institute Medical Sciences New Delhi, for providing the research facilities, for recording TEM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ali, S.G., Ansari, M.A., Khan, H.M. et al. Antibacterial and Antibiofilm Potential of Green Synthesized Silver Nanoparticles against Imipenem Resistant Clinical Isolates of P. aeruginosa. BioNanoSci. 8, 544–553 (2018). https://doi.org/10.1007/s12668-018-0505-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0505-8