Abstract
Cobalt oxide nanoparticles, Co3O4 (1) and Co3O4 (2), have been synthesized by thermal decomposition of [CoII(bqbenzo)] and [CoII(bqb)], respectively. The morphology of these oxides is influenced by the difference in the structure of bqbenzo2− {3,4-bis(2-quinolinecarboxamido) benzophenone and, bqb2− {bis(2-quinolinecarboxamido)-1,2-benzen}, only differing in a benzoyl substituent. The products were characterized by XRD, FE-SEM, and FT-IR spectroscopy. The catalytic activity of the oxides was examined in oxygen evolution reaction (OER) by cyclic voltammetry (CV) and linear sweep voltammetry (LSV). The Co3O4 oxides (1 and 2) exhibited higher catalytic activity compared to 10 wt% Pt/C in terms of obtained current density at 0.8 V; ∼23.3 versus 6.1 mA cm−2, respectively. However, the aging tests of the two oxides in OER revealed that Co3O4 (1) is more stable than Co3O4 (2). These results demonstrated that the Co3O4 (1) has a superior performance which can be employed in the alkaline water electrolyzer anode.

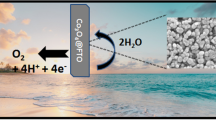
Co3O4 nanoparticles are synthesized via calcination of [Co(bqbenzo)] and [Co(bqb)] giving Co3O4 (1) and Co3O4 (2), respectively. Both oxides exhibit pronounced oxygen evolution activity compared to 10 wt% Pt/C and Co3O4 (1), with more robust polymorphic structure, exhibits remarkable stability during OER cycling.








Similar content being viewed by others
References
P. Du, R. Eisenberg, Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: recent progress and future challenges. Energy Environ. Sci. 5, 6012–6021 (2012)
L. Trotochaud, J. K. Ranney, K. N. Williams, S. W. Boettcher, Solution-cast metal oxide thin film electrocatalysts for oxygen evolution. J. Am. Chem. Soc. 134, 17253–17261 (2012)
S. M. Barnett, K. I. Goldberg, J. M. Mayer, A soluble copper-bipyridine water-oxidation electrocatalyst. Nat. Chem. 4, 498–502 (2012)
M. Garcia-Mota, M. Bajdich, V. Viswanathan, A. Vojvodic, A. T. Bell, J. K. Norskov, Importance of correlation in determining electrocatalytic oxygen evolution activity on cobalt oxides. J. Phys. Chem. C116, 21077–21082 (2012)
L. Duan, F. Bozoglian, S. Mandal, B. Stewart, T. Privalov, A. Llobet, L. Sun, A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nat. Chem. 4, 418–423 (2012)
Y. Surendranath, M. W. Kanan, D. G. Nocera, Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J. Am. Chem. Soc. 132, 16501–16509 (2010)
M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori, N. S. Lewis, Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010)
Y. Lee, J. Suntivich, K. J. May, E. E. Perry, Y. Shao-Horn, Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 3, 399–404 (2012)
M. W. Louie, A. T. Bell, An investigation of thin-film Ni-Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 135, 12329–12337 (2013)
N. H. Chou, P. N. Ross, A. T. Bell, T. D. Tilley, Comparison of cobalt-based nanoparticles as electrocatalysts for water oxidation. ChemSusChem 4, 1566–1569 (2011)
T. Maiyalagan, K. A. Jarvis, S. Therese, P. J. Ferreira, A. Manthiram, Spinel-type lithium 316 cobalt oxide as a bifunctional electrocatalyst for the oxygen evolution and oxygen 317 reduction reactions. Nat. Commun. 5(3949), 1–8 (2014)
N. Suzuki, T. Horie, G. Kitahara, M. Murase, K. Shinozaki, Y. Morimoto, Novel noble-metal-free electrocatalyst for oxygen evolution reaction in acidic and alkaline media. Electrocatalysis 7, 115–120 (2016)
M. Dincă, Y. Surendranath, D. G. Nocera, Nickel-borate oxygen-evolving catalyst that functions under benign conditions. Proc. Natl. Acad. Sci. 107, 10337–10341 (2010)
K. Fominykh, J. M. Feckl, J. Sicklinger, M. Döblinger, S. Böcklein, J. Ziegler, L. Peter, J. Rathousky, E.-W. Scheidt, T. Bein, D. Fattakhova-Rohlfing, Ultrasmall dispersible crystalline nickel oxide nanoparticles as high-performance catalysts for electrochemical water splitting. Adv. Funct. Mater. 24, 3123–3129 (2014)
H. Wang, H.-W. Lee, Y. Deng, Z. Lu, P.-C. Hsu, Y. Liu, D. Lin, Y. Cui, Bifunctional non-noble metal oxide nanoparticle electrocatalysts through lithium-induced conversion for overall water splitting. Nat. Commun. 6(7261), 1–8 (2015)
R. K. Gupta, A. K. Sinha, B. N. Raja Sekhar, A. K. Srivastava, G. Singh, S. K. Deb, Synthesis and characterization of various phases of cobalt oxide nanoparticles using inorganic precursor. Appl. Phys. A Mater. Sci. Process. 103, 13–19 (2011)
K. Thangavelu, K. Parameswari, K. Kuppusamy, Y. Haldorai, A simple and facile method to synthesize Co3O4 nanoparticles from metal benzoate dihydrazinate complex as a precursor. Mater. Lett. 65, 1482–1484 (2011)
M. Goudarzi, M. Bazarganipour, M. Salavati-Niasari, Synthesis, characterization and degradation of organic dye over Co3O4 nanoparticles prepared from new binuclear complex precursors. RSC Adv. 4, 46517–46520 (2014)
O. Belda, C. Moberg, Bispyridylamides–coordination chemistry and applications in catalytic reactions. Coord. Chem. Rev. 249, 727–740 (2005)
L. Yang, Z. Wu, L. Liang, X. Zhou, Synthesis, crystal structures and catalytic abilities of new macrocyclic bis-pyridineamido MnIII and FeIII complexes. J. Organomet. Chem. 694, 2421–2426 (2009)
D. H. Lee, J. H. Lee, B. K. Park, E. Y. Kim, Y. Kim, C. Kim, I. M. Lee, High catalytic activities in the norbornene polymerization with neutral palladium complexes containing N4-type tetradentate chelating ligands. Inorg. Chim. Acta 362, 5097–5102 (2009)
C. Y. Shi, E. J. Gao, S. Ma, M. L. Wang, Q. T. Liu, Synthesis, crystal structure, DNA-binding and cytotoxicity in vitro of novel cis-Pt(II) and trans-Pd(II) pyridine carboxamide complexes. Bioorg. Med. Chem. 20, 7250–7254 (2010)
K. Sakai, H. Ozawa, H. Yamada, T. Tsubomura, M. Hara, A. Higuchi, M. A. Haga, A tris(2,2′-bipyridine)ruthenium(II) derivative tethered to a cis-PtCl2(amine)2 moiety: syntheses, spectroscopic properties, and visible-light-induced scission of DNA. J. Chem. Soc. Dalton Trans., 3300–3305 (2006)
R. D. Litto, V. Benessere, F. Ruffo, C. Moberg, Carbohydrate-based pyridine-2-carboxamides for Mo-catalyzed asymmetric allylic alkylations. Eur. J. Org. Chem., 1352–1356 (2009)
R. Ramachandran, P. Viswanathamurthi, Ruthenium(II) carbonyl complexes containing pyridine carboxamide ligands and PPh3/AsPh3/Py coligands: synthesis, spectral characterization, catalytic and antioxidant studies. Spectrochim. Acta Part A 103, 53–61 (2013)
D. N. Lee, J. Y. Ryu, H. Kwak, Y. M. Lee, S. H. Lee, J. I. Poong, J. Lee, W. Shin, C. Kim, S. J. Kim, Y. Kim, Steric effect on construction of Cu(II) complexes with pyridine carboxamide ligands. J. Mol. Struct. 885, 56–63 (2008)
A. A. Eroy-Reveles, Y. Leung, C. M. Beavers, M. M. Olmstead, P. K. Mascharak, Near-infrared light activated release of nitric oxide from designed photoactive manganese nitrosyls: strategy, design, and potential as NO donors. J. Am. Chem. Soc. 130, 4447–4458 (2008)
H. A. Zamani, R. Kamjoo, M. Mohammadhosseini, M. Zaferoni, Z. Rafati, M. R. Ganjali, Farnoush Faridbod, Soraia Meghdadi, Europium(III) PVC membrane sensor based on N-pyridine-2-carboxamido-8-aminoquinoline as a sensing material. Mater. Sci. Eng. C 32, 447–451 (2012)
S. Meghdadi, K. Mereiter, M. Amirnasr, F. Karimi, A. Amiri, Synthesis, crystal structure and electrochemistry of cobalt(III) carboxamide complexes with amine and azide ancillary ligands. Polyhedron 68, 60–69 (2014)
S. Meghdadi, M. Amirnasr, A. Amiri, Z. Musavizadeh Mobarakeh, Z. Azarkamanzad, Benign synthesis of N-(8-quinolyl pyridine-2-carboxamide) ligand (Hbpq), and its Ni(II) and Cu(II) complexes. A fluorescent probe for direct detection of nitric oxide in acetonitrile solution based on Hbpq copper(II) acetate interaction. C. R. Chim 17, 477–483 (2014)
S. Meghdadi, M. Amirnasr, A. Amiri, Z. Azarkamanzad, K. Schenk Joβ, F. Fadaee, A. Amiri, S. Abbasi, Benign synthesis of the unsymmetrical ligand N-(quinolin-8-yl) pyrazine-2-carboxamide. Preparation, electrochemistry, antibacterial activity, and crystal structures of Cu(II) and Zn(II) complexes. J. Coord. Chem. 66, 4330–4343 (2013)
M. Salavati-Niasari, F. Davar, M. Mazaheri, M. Shaterian, Preparation of cobalt nanoparticles from [bis(salicylidene)cobalt(II)]–oleylamine complex by thermal decomposition. J. Magn. Magn. Mater. 320, 575–578 (2008)
M. Herrero, P. Benito, F. M. Labajos, V. Rives, Nanosize cobalt oxide-containing catalysts obtained through microwave-assisted methods. Catal. Today 128, 129–137 (2007)
R. Jenkins, R. L. Snyder, Chemical analysis: introduction to X-ray powder diffractometry (Wiley, Inc., New York, 1996), p. 90
X. Wu, K. Scott, A Li-doped Co3O4 oxygen evolution catalyst for nonprecious metal alkaline anion exchange membrane water electrolysers. Int. J. Hydrogen Energ. 38, 3123–3129 (2013)
B. Chi, H. Lin, J. Li, Cations distribution of CuxCo3-xO4 and its electrocatalytic activities for oxygen evolution reaction. Int. J. Hydrogen Energ. 33, 4763–4768 (2008)
M. Hamdani, R. N. Singh, P. Chartier, Co3O4 and Co-based spinel oxides bifunctional oxygen electrodes. Int. J. Electrochem. Sci. 5, 556–577 (2010)
Y.-C. Liu, J. A. Kza, J. A. Switzer, Conversion of electrodeposited Co(OH) to CoOOH and Co3O4, and comparison of their catalytic activity for the oxygen evolution reaction. Electrochim. Acta 140, 359–365 (2014)
Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier, H. Dai, Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nature Mater. 10, 780–786 (2011)
Acknowledgements
Partial support of this work by the Isfahan University of Technology Research Council and the Iranian Nano Technology Initiative Council is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
ESM 1
(DOCX 789 kb)
Rights and permissions
About this article
Cite this article
Meghdadi, S., Amirnasr, M., Zhiani, M. et al. Facile Synthesis of Cobalt Oxide Nanoparticles by Thermal Decomposition of Cobalt(II) Carboxamide Complexes: Application as Oxygen Evolution Reaction Electrocatalyst in Alkaline Water Electrolysis. Electrocatalysis 8, 122–131 (2017). https://doi.org/10.1007/s12678-016-0345-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-016-0345-7