Abstract
A new coagulant obtained through polymerization of Acacia mearnsii de Wild tannin extract has been characterized in the removal of two dangerous dye pollutants: Alizarin Violet 3R and Palatine Fast Black WAN. This coagulant is lab-synthesized according to the etherification of tannins with glycidyltrimethylammonium chloride and formaldehyde and its performance in dye removal in terms of efficiency was high. Reasonably low coagulant dosages (ca. 50 mg L−1) reaches high capacity levels (around 0.8 for Alizarin Violet 3R and 1.6 for Palatine Fast Black WAN mg dye mg−1 of coagulant) and pH and temperature are not extremely affecting variables. The systems coagulant dyes were successfully modeled by applying the Langmuir hypothesis. qmax and b parameters were obtained with an adjusted correlation factor (r2) above 0.8.
Similar content being viewed by others
Introduction
The industrial production of dyes has become a highly contaminant activity in the recent decades. Dyes are worldwide used for many different scopes: from textile to pharmaceutical uses, the global demand of dyes is obviously linked to the general disposal of effluents with colorants that for sure affect environmental equilibrium. The exponential growth of some kinds of industries in so-called developing countries have made these contaminants remarkably influent in emerging areas (Harleman et al. 1999; Robinson et al. 2001). Many authors have already advised about the fragility of the environment (Ryan and Elimelech 1996) and the challenge of cheaper and affordable water treatment processes, specially indicated for dye removal, is still active (Wang et al. 2007).
Anionic dyes are the most popular colorants and they are used in a large variety of applications, such as textiles, paper, foodstuffs and cosmetics (Shore 2002). Alizarin Violet 3R is a relevant and paradigmatic dye of this group, and it is usually presented as a model compound (Sánchez-Martín et al. 2010a). This is an anthraquinonic, synthetic dye. It is characterized by a high chemical and biological oxygen demand and intense violet color (Zollinger 1987). These aspects make industrial effluents of this dye highly toxic and extremely injurious to both aquatic and life forms. The difficulty met when attempting to degrade or remove this dye has been thoroughly reported previously (Robinson et al. 2001) and is mainly caused by the five aromatic rings and the two sulfonated groups that make this dye a persistent and carcinogenic agent. Its chemical structure is showed in Fig. 1.
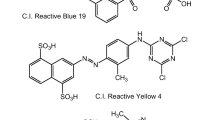
On the other hand, Palatine Fast Black WAN is an azo dye which also belongs to this group of anionic colorants. It is an extremely long molecule whose structure includes 12 aromatic rings and 3 sulfonate groups, apart from many other functional groups (Fig. 2). The presence of chromium atoms associated with the organic chain makes it specially dangerous, attending to its mutagenic action (Chung et al. 2006).
The removal of dyes from aqueous solutions can be carried out through several chemical and/or physical methods. One of the most popular processes in water treatment is coagulation (Lee et al. 2006). It is considered a chemical treatment as it implies the addition of a coagulant. Stable colloids in water normally present negative charges all around their surface. Coagulant is able to cause the neutralization of these charges, so colloidal particles become unstable and tend to settle by gravity (Kim 1995). Typical coagulant agents are inorganic salts, such as Al2(SO4)3 or FeCl3, as well as synthetic polyacrylamides (Papic et al. 2004). Although these chemicals are rather effective in removing dyes and suspended matter from the aqueous matrix, several disadvantages have recently arisen, such as their impact on human diseases like Alzheimer’s (Flaten 2001) or cancer. These suspicions have already forced the removal of polyacrylamides from drinking water treatment plants in many countries in accordance with the suggestions of the World Health Organization (WHO 2003).
New coagulant agents are needed therefore to overcome the drawbacks that traditional chemicals seem to presents. In this scenario, tannins are presented as a promising source for new coagulant agents. Tannins are mostly vegetal water-soluble polyphenolic compounds with molecular weight ranged between 500 and some thousand Daltons. Trees, such as Schinopsis balansae (Quebracho), Castanea sativa (Chestnut) or Acacia mearnsii de Wild (Black wattle) are well-known tannin sources. From a chemical point of view, there are three kinds of tannins: hydrolyzable, condensed and combined ones (Haslam 1989). These products are rather chemically complex and they are usually taken from a natural matrix, without a very exhaustive purification. Because of that, it is rather difficult to know their structure exactly although probable approaches can be found in specific literature (Fig. 3).
Regarding water treatment, tannins can be used in two main ways: jellified and cationized. Although the jellification is used for adsorption of cationic pollutants in water (Nakano et al. 2001; Kim and Nakano 2005; Tondi et al. 2009; Beltrán-Heredia et al. 2011a), tannin cationization may drive to a new coagulant that is able to remove anionic compounds. The chemical procedure of cationization can follow a Mannich reaction path and different variations were reported under several patents (Quamme and Kemp 1985; Vasconcellos et al. 1993; Reed and Finck 1997; Mitchel et al. 1998). The scientific literature refers a reaction mechanism that involves a tannin mixture whose structure may be similar to flavonoid structures, such as resorcinol A and pyrogallol B rings (Fig. 3). In brief, Mannich reaction is described as the introduction of a quaternary nitrogen inside the tannin complex structure (Tramontini and Angiolini 1994). Tannins undergo Mannich aminomethylation by reaction with an aldehyde and an amine (Roux et al. 1975). Etherification is another possibility of this kind of chemical modification, although it is mainly used in epoxy resin systems (Shechter and Wynstra 1956). In a general way, phenol-glycidyl ether reaction occurs in basic medium and involves the opening of epoxy group in order to attack directly onto the hydroxyl group, as can be appreciated in Fig. 4. The resulting tannin polymer possesses higher molecular weight due to formaldehyde and the subsequent crosslinking. Ampholytic character is also obtained due to the presence of both cationic amines and anionic phenols on the polymer. Pollutants with anionic electrical charge are directly removed by this product.
This investigation is focused in advanced water treatment through a new coagulation process that is (1) cheaper than others, (2) based on a natural product and (3) easy to handle and maintain for unskilled personnel. Environmental equilibrium at global level may need us to make the possibility of becoming clean a universal chance.
Materials and methods
Coagulant syntheses
The reagents involved in the cationization process are
-
1.
Tannin extracts were kindly supplied by TANAC Inc. (Brazil) and Raoul-Duvall Inc. (France). They were commercial sources for tannin reagents involved in leather tanning and specifications are shown in Table 1.
-
2.
Glycidyltrimethylammonium chloride and formaldehyde were both supplied by SIGMA in commercial purity grade.
According to the previous data (Beltrán-Heredia et al. 2010), the cationization processes were conducted as follows:
-
2.5 g amount of tannin extract was diluted in distilled water at room temperature. Then, the sample was thermostated at the reaction temperature (30°C).
-
2.5 g of the ammonium compound was added to the mix.
-
Always under thermal control, 0.23 g of pure formaldehyde was added to the reaction mixture. This corresponds to 5 mL of the commercial solution. A peristaltic pump (Masterflex, COLEPARMER) must be used in this step, so it lasts for 90 min at least.
-
The product so obtained must be kept under agitation and at the same temperature for 24 h.
The final product was then put in a 50 mL flask and filled up to the mark with distilled water.
Buffered solution
The trials with added dye were performed with pH-stable media according to preliminary data (Beltrán-Heredia et al. 2009a). To this end, a pH 7 buffer solution was prepared by mixing 1.2 g of NaH2PO4 and 0.885 g of Na2HPO4 in 1 L flask and filled to the mark with distilled water. The pH was then adjusted to 7 with HCl 0.5 M and NaOH 0.5 M. All reagents were analytical grade from PANREAC.
Model compounds
Two main dyes were used as model compounds:
-
Alizarin Violet 3R (CI 61710) was supplied by SIGMA-ALDRICH. It is an anthraquinonic dye (C28H20N2Na2O8S2) with molecular weight equal to 622.6 g mol−1.
-
Palatine Fast Black WAN (CI 15711) was also supplied by SIGMA-ALDRICH. It is an azo dye (C60H36N9Na3O21S3. Cr2) with molecular weight equal to 1,448 g mol−1.
Apart from these, other dyes were included in the preliminary screening. Their chemical structures are included in Supplementary data.
General dye removal trials
A dye solution of 1,000 mg L−1 was prepared. This was used as stock solution. Aliquots of 100 mL of this simulated wastewater were mixed with the equivalent dose of coagulant for each case to reach the adequate concentration of dye and coagulant. Stirring at 30 rpm for 1 h was applied, until equilibrium was achieved. Then, a sample was taken and it was centrifuged. Photometric analysis was carried out in a 1-cm glass cell at maximum absorbance for each dye.
In the case of Alizarin Violet 3R and Palatine Fast Black WAN, kinetics of dye removal were experimentally confirmed. As Fig. 5 shows two different solutions of ca. 100 mg L−1 of each dye were treated with 20 and 40 mg L−1 of coagulant each one and the response were stable enough to consider the coagulation almost immediate. This aspect is well known and it was previously presented in other papers (Beltrán-Heredia et al. 2009a).
Results and discussion
The study was carried out in three complementary dimensions: first a preliminary screening of dye removal was performed using the working lab-made coagulants. Then, the influence of operative factors, such as pH or initial dye concentration (IDC) was determined for the specific removal of Alizarin Violet 3R and Palatine Fast Black WAN. Finally, both dye–coagulant systems were theoretically modeled according to Langmuir hypothesis.
Preliminary screening for dye removal
A preliminary screening is performed to evaluate the removal ability of four lab-made coagulants. Each one is synthesized from a different tannin source: black wattle (CTC), chestnut (ChTC), quebracho (QTC) and tara (TTC). They are tested on the removal of 11 model dyes, as can be seen in Fig. 6. It depicts the percentage removal in a standarized trial with 100 mg L−1 of dye and 200 mg L−1 of coagulant, pH 7 and 20°C.
Different groups of dyes are considered in this evaluation. Although Alizarin Violet 3R is an anthraquinonic dye, azo dyes are represented by six of these compounds: Direct Red 28, Palatine Fast Black WAN, New Coccine, Chicago Sky Blue 6B, Tartrazine and Amaranth. Carmine Indigo is the only sample for indigoid ones, and Erioglaucine, Patent Blue V and Eriochromecyanine R belong to triphenylmethane group. The performance of the coagulants differs mainly due to the tannin nature and the dye chemical structure and corresponding type.
Regarding the different coagulants, it is clear that condensed tannins presented an efficient performance, while hydrolyzable ones were less effective (TTC and ChTC). The cationization process is, therefore, more convenient with the first group of tannin sources, as it is previously reported in scientific literature (Beltrán-Heredia et al. 2010; 2011a). Among condensed tannins (CTC and QTC) in almost every case black wattle presents a higher performance than quebracho-derived tannin coagulant. The reason of this difference may be found on the lower reactivity of the quebracho constitutive flavonoids (Streit and Fengel 1994; Schofield et al. 2001) in comparison with Acacia ones. Because of this fact black wattle is nowadays the first source of tannins.
Regarding, on the other hand, the type of colorant, there are evidences of the higher response with azo dyes. The percentual removal of Chicago Sky Blue 6B, Direct Red 28 or Palatine Fast Black WAN (all of them azo dyes) is significant, while Alizarin Violet 3R or Erioglaucine presented not so high removal. The most refractory dyes are Tartrazine and Patent Blue V, due surely to their electrical polarity or even to steric difficulties in the coagulation process.
Owing to the worldwide presence and their special pollutant activity, two dyes are selected for further study: Alizarin Violet 3R and Palatine Fast Black WAN. The selected coagulant is the one derived from Acacia mearnsii de Wild, Clarotan Tannin Coagulant (CTC), although the performance of these coagulants with the rest of the dyes are promising.
Operative factors
In agreement with the usual description of influent variables coagulant dosage, pH and IDC were evaluated as operative factors. To evaluate not only the efficacy of the treatment, but also the efficiency, dye removal must be complemented by other objective target. Several previous works have pointed out the adecuacy of adsorption capacity (q) also for this scope (Sánchez-Martín et al. 2010a; Beltrán-Heredia et al. 2009a; Beltrán-Heredia and Sánchez-Martín 2009). Adsorption capacity (q, mg mg−1) was determined according to the following equation (1):
where
C0 is initial contaminant concentration (mg L−1),
C l is equilibrium contaminant concentration in bulk solution (mg L−1),
V is the volume of solution (L), and W is coagulant mass (mg).
Coagulant dose
The coagulant ability for dye removal is clearly presented in Fig. 7. Different experiments with equal initial dye concentration (ca. 100 mg L−1) were subsequently treated with increasing doses of coagulant. As can be appreciated, reasonably low doses of coagulant (around 100 mg L−1) can easily remove almost 50% of the dye concentration, although a qualitative difference is observed between both systems.
Both subfigures present similar performances of dye removal and the decrease in colorant content is quite comparable. The coagulant capacities are observed inside a very interesting range, an initial q is set up to 1 mg mg−1, which is a high ratio (Beltrán-Heredia and Sánchez-Martín 2008; Beltrán-Heredia et al. 2011b). It is, however, significative the first low capacity level in the early doses of CTC for Palatine Fast Black WAN removal. This may have to do with the presence of a minimum coagulant concentration for initiate the coagulant process (Beltrán-Heredia et al. 2010) and until it is not reached the removal of dye does not begin.
pH
Several experiments with different pH values have been carried out, varying the pH between 4 and 9, with an IDC of ca. 100 mg L−1 and with a fixed coagulant dosage of 40 mg L−1. Figure 8 shows the experimental results under the form of dye removal (%) versus pH. As it is reported, a significant decreasing tendency is presented as pH level raises. This behavior was also observed in our previous works (Sánchez-Martín et al. 2009, 2010b). This may be linked to the structure of the tannin (Fig. 3) which probably undergoes a partial denaturation because of the attack of hydroxyl groups (OH−). In this case, there is no clear difference between both systems, even in the level of influence of pH on the dye removal and coagulant activity.
Temperature
Both systems seem to present stability along the studied temperature range (10–40°C). Initial dye concentrations of 100 mg L−1 were treated with a fixed coagulant dose of 50 mg L−1, pH 7 and different temperatures. As can be observed in Fig. 9, no significant differences can be stated from these experimental series. In a general way, temperature does not seem to be significatively important. This stability adds a new advantage to the studied coagulant, since it allows the treatment of wastewater under thermal contamination, e.g. lakes or ponds, which is a desirable characteristic (Mohan et al. 2002).
Initial dye concentration
It was also considered important to research on how significant IDC was with reference to the percentage of dye removal in each case and, in addition, to the q level. A series of experiments were carried out by varying only the initial dye concentration between 50 and 500 mg L−1. Equal coagulant doses of 100 mg L−1 were applied and the results are clearly shown in Fig. 10. It is more than obvious that increasing the IDC leads to a loss of the percentage of dye removal, but the most interesting point is the fact that q tends to be higher as IDC raises and finally keeps around the maximum q (as calculated in section 3), 0.87 mg mg−1 in the case of Alizarin Violet 3R and 1.21 mg mg−1 in the case of Palatine Fast Black WAN.
Mathematical modeling
The physical system dye-coagulant may be ruled by adsorption-like relationships. Taking into consideration the need for characterizing how this coagulant works on Alizarin Violet 3R or Palatine Fast Black WAN removal, a model must be proposed to determine the best way of scaling-up this new treatment agent. To this end, one must define an target variable that should be referred to the removal efficiency for the specific contaminant. Consequently, it must link the percentage removal and the amount of coagulant.
Coagulation and flocculation processes are rather difficult to model mathematically, due to two main reasons: (1) the complex nature of the phenomenon, which implies physico-chemical interaction molecule–molecule (van der Waals and hydrogen bridges forces) (Wilkinson et al. 1997) and (2) the fact that the intrinsic composition of the organic material that forms the flocculant active principle is not completely known.
The parameter extensively used in adsorption processes is the adsorption capacity, q. Our working hypothesis was that contaminant removal by coagulation and flocculation occurs in two stages. First, there is destabilization of colloids which may be governed by chemical interactions between molecules of the coagulant (cationic, positively charged) and of the contaminant (anionic, negatively charged). Then, once the coagulant–contaminant complex is formed, flocs begin to grow by sorption mechanisms. This should be the controlling stage, so that the entire process can be simulated as an adsorption phenomenon. Previous studies have found the coagulation capacity q to be a suitable evaluation parameter (Beltrán-Heredia et al. 2009b).
Although other hypothesis are feasible (Freundlich 1906) the main adsorption (and coagulation) model is the one Langmuir presented in the early years of the twentieth century (Langmuir 1916). The fact that it is theoretically deduced makes it still feasible and appropriate. Probability of adsorption rate is proportional to the number of active sites, whereas probability of desorption is proportional to the number of already occupied sites. Those probabilities are related to the strenght of the interaction between the adsorbent surface and the adsorbate. That is the physical meaning of the Eq. (2):
where
qmax is the maximum q capacity (mg of dye [mg of coagulant]−1),
and b is the Langmuir adsorption constant ([mg of dye] L−1).
The linear form of Langmuir model can be expressed by Eq. 3:
Figure 11 presents the experimental data of these equilibrium systems and the predicted ones by Langmuir model, according to Eq. 2. The statistical summary of these mathematical data adjustments is presented in Table 2. The nonlinear Langmuir fits for the specific temperatures give lower correlation coefficients than linear ones because the error bars are wider in this case. However, previous studies have shown this method to be more accurate than linear fits since no specific conditions regarding normal distribution of errors must be taken into consideration (Kumar and Sivanesan 2006; Kumar et al. 2008).
As can be appreciated at a first glance, a very good fit is presented in both cases. Numerical non-linear fit gives us an accurate adjusted r2 (0.94 and 0.81) and the values of qmax and b are also reliable with the previous data of IDC influence. The consistency of the whole study is reinforced by the fact that maximum capacity predicted by Langmuir’s model is very similar to the general tendency of q when the working variable is the initial dye concentration, hence the empirical confirmation of this mathematical model.
Conclusions
Tannin-derived coagulants are a satisfactory new agents for water treatment. Specially tannins from Acacia mearnsii de Wild are easily cationized with glycidyltrimethyl ammonium chloride and formaldehyde and the application of this novel product on dye removal is very satisfactory with several types of dyes: azo, anthraquinonic, indigoid and triphenylmethane ones. Particularly Palatine Fast Black WAN and Alizarin Violet 3R are very reactive to this coagulation and flocculation process, and the purification of these kinds of effluents is feasible in a wide range of conditions, including pH, temperature and different colorant pollution. The interaction system between coagulant and dye can be modeled according to Langmuir hypothesis, and the maximum q levels are very high: 0.87 and 1.21 \(\hbox{mg}\,{\rm mg}^{-1}\) for Alizarin Violet 3R and Palatine Fast Black WAN, respectively.
References
Beltrán-Heredia J, Sánchez-Martín J (2008) Azo dye removal by Moringa oleifera seed extract coagulation. Color Technol 124(5):310–317
Beltrán-Heredia J, Sánchez-Martín J (2009) Removal of sodium lauryl sulphate by coagulation/flocculation with Moringa oleifera seed extract. J Hazard Mater 164(2–3):713–719
Beltrán-Heredia J, Sánchez-Martín J, Dávila-Acedo MA (2011) Optimization of the synthesis of a new coagulant from a tannin extract. J Hazard Mater 186(2–3):1704–1712
Beltrán-Heredia J, Sánchez-Martín J, Delgado-Regalado A, Jurado-Bustos C (2009a) Removal of Alizarin Violet 3R (anthraquinonic dye) from aqueous solutions by natural coagulants. J Hazard Mater 170(1):43–50
Beltrán-Heredia J, Sánchez-Martín J, Gómez-Muñoz MC (2010) New coagulant agents from tannin extracts: preliminary optimisation studies. Chem Eng J 162:1019–1025
Beltrán-Heredia J, Sánchez-Martín J, Martín-Sánchez C (2011b) Remediation of dye-polluted solutions by a new tannin-based coagulant. Indus Eng Chem Res 50(2):686–693
Beltrán-Heredia J, Sánchez-Martín J, Solera-Hernández C (2009b) Anionic surfactants removal by natural coagulant/flocculant products. Indus Eng Chem Res 48(10):5085–5092
Chung K-T, Chen S-C, Claxton LD (2006) Review of the Salmonella typhimurium mutagenicity of benzidine, benzidine analogues, and benzidine-based dyes. Mutat Res Rev Mutat Res 612(1):58–76
Flaten P (2001) Aluminium as a risk factor in Alzheimer’s disease, with emphasis in drinking water. Brain Res Bull 55(2):187–196
Freundlich H (1906) Uber die adsorption in losungen. Zeitschrift fur Physikalische Chemie 57:385–470
Harleman DRF, Murcott S (1999) The role of physical–chemical wastewater treatment in the mega-cities of the Developing World. Water Sci Technol 40(4–5):75–80
Haslam E (1989) Plant polyphenols-vegetables and tannins revisited. Cambridge University Press, Cambridge
Kim Y-H, Nakano Y (2005) Adsorption mechanism of palladium by redox within condensed-tannin gel. Water Res 39(7):1324–1330
Kim YH (1995) Coagulants and flocculants. Tall Oak Publishing, Littleton
Kumar KV, Sivanesan S (2006) Pseudo second order kinetics and pseudo isotherms for malachite green onto activated carbon: comparison of linear and non-linear regression methods. J Hazard Mater 136(3):721–726
Kumar KV, Porkodi K, Rocha F (2008) Isotherms and thermodynamics by linear and non-linear regression analysis for the sorption of methylene blue onto activated carbon: comparison of various error functions. J Hazard Mater 151(2–3):794–804
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids . J Am Chem Soc 38:2221–2295
Lee J-W, Choi S-P, Thiruvenkatachari R, Shim W-G, Moon H (2006) Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigments 69(3):196–203
Mitchel DB, Minnis RL, Curran TP, Deboo SM, Kelly JA, Patwardhan R, Tai W-T (1998) Treatment of aqueous systems using a chemically modified tannin. US Patent 5,843,337
Mohan SV, Chandrasekhar N, Prasad K, Karthikeyan J (2002) Treatment of simulated reactive Yellow 22 (Azo) dye effluents using Spirogyra species. Waste Manag 22(6):575–582
Nakano Y, Takeshita K, Tsutsumi T (2001) Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Res 35(2):496–500
Papic S, Koprivanac N, Bozic AL, Metes A (2004) Removal of some reactive dyes from synthetic wastewater by combined Al(III) coagulation/carbon adsorption process. Dyes Pigments 62(3):291–298
Quamme JE, Kemp AH (1985) Stable tannin based polymer compound. US Patent 4,558,080
Reed PE, Finck MR (1997) Modified tannin mannich polymers. US Patent 5,659,002
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77(3):247–255
Roux DG, Ferreira D, Hundt HL, Malan E (1975) Structure, stereochemistry, and reactivity of natural condensed tannins as basis for their extended industrial application. Appl Polym Symp 1(28):335–353
Ryan JN, Elimelech M (1996) Colloid mobilization and transport in groundwater. Colloids Surfaces A Physicochem Eng Aspects 107:1–56
Sánchez-Martín J, Beltrán-Heredia J, Solera-Hernández C (2010a) Surface water and wastewater treatment using a new tannin-based coagulant. Pilot plant trials. J Environ Manag 91:2051–2058
Sánchez-Martín J, González-Velasco M, Beltrán-Heredia J (2009) J Wood Chem Technol 29(2):119–135
Sánchez-Martín J, González-Velasco M, Beltrán-Heredia J (2010b) Surface water treatment with tannin-based coagulants from Quebracho (Schinopsis balansae). Chem Eng J 165:851–858
Schofield P, Mbugua DM, Pell AN (2001) Analysis of condensed tannins: a review. Anim Feed Sci Technol 91(1):21–40
Shechter L, Wynstra J (1956) Glycidyl ether reactions with alcohols, phenols, carboxylic acids, and acid anhydrides. Indus Eng Chem 48(1):86–93
Shore J (2002) Colorants and auxiliaries: organic chemistry and application properties, 2nd edn. Bradford
Streit W, Fengel D (1994) Purified tannins from quebracho colorado. Phytochemistry 36(2):481–484
Tondi G, Oo CW, Pizzi A, Thevenon MF (2009) Metal absorption of tannin-based rigid foams. Indus Crops Products 29(2–3):336–340
Tramontini M, Angiolini L (1994) Mannich bases. CRC Press, Boca Raton
Vasconcellos SR, Boyce PD, Smith LP (1993) Methods for the flocculation of coal fines and insoluble metals in coal mine waters. US Patent 4,183,575
Wang J-P, Chen Y-Z, Ge X-W, Yu H-Q (2007) Optimization of coagulation-flocculation process for a paper-recycling wastewater treatment using response surface methodology. Colloids Surf A Physicochem Eng Aspects 302(1–3):204–210
WHO (2003) Acrylamide in drinking water. World Health Organization, WHO/SDE/WSH/03.04.71f
Wilkinson KJ, JC Negre JC, Buffle J (1997) Coagulation of colloidal material in surface waters: the role of natural organic matter. J Contam Hydrol 26(1–4):229–243
Zollinger H (1987) Colour chemistry-synthesis, properties and application of organic dyes and pigments. VCH Publishers, New York
Acknowledgments
This investigation has been supported by the COMISIÓN INTERMINISTERIAL DE CIENCIA Y TECNOLOGÍA (CICYT) CTQ 2010-14823/PPQ project as well by JUNTA DE EXTREMADURA under PRI-07A031 project.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Beltrán-Heredia, J., Sánchez-Martín, J. & Rodríguez-Sánchez, M.T. Textile wastewater purification through natural coagulants. Appl Water Sci 1, 25–33 (2011). https://doi.org/10.1007/s13201-011-0005-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-011-0005-2