Abstract
A preliminary study was conducted for the removal of turbidity (TD), chemical oxygen demand (COD) and biochemical oxygen demand (BOD) from secondarily treated sewage (STS) water through the electrolytic batch mode experiments with DC power supply (12 V) up to 30 min and using a novel concept of electrode combinations of different metals. The different surface areas (40, 80, 120 and 160 cm2) of the electrodes as a function of cross-sectional area of the reactor and the effect of inter-electrode distances (2.5–10 cm) on the electrolysis of STS water were studied. This study revealed that the effluent can be effectively treated with the aluminum (Al) and iron (Fe) electrode combinations (Al–Fe and Fe–Al). The maximum removal of TD (81.51 %), COD (74.36 %) and BOD (70.86 %) was recorded with Al–Fe electrode system, while the removal of these parameters was found to be 71.11, 64.95 and 61.87 %, respectively, with Fe–Al electrode combination. The Al–Fe electrode combination had lower electrical energy consumption (2.29 kWh/m3) as compared to Fe–Al electrode combination (2.50 kWh/m3). The economic evaluation of electrodes showed that Al–Fe electrode combination was better than Fe–Al electrode combination. This revealed the superiority of aluminum as a sacrificial electrode over that of iron which can probably be attributed to better flocculation capabilities of aluminum than that of iron.
Similar content being viewed by others
Introduction
Water is an essential substance for living system as it allows the transport of nutrients as well as waste products in the living systems. However, sustainable water supply is becoming more challenging by the day due to ever increasing demand of growing population as well as increasing contamination of water resources. At the same time, huge quantities of wastewater generated by industries of every hue and kind and also by exponential growth in the number of households are becoming a serious concern for society.
The role of electrochemistry in water and effluent treatment is relatively small, since conventional electrode materials achieve only low current efficiencies due to the water electrolysis side reactions (Comninellis 1994; Simonsson 1997). However, the use of sacrificial electrodes of metals which can give rise to multiple charged ions and their corresponding salts in the electrolytic systems results in coagulation and flocculation of dissolved and undissolved water impurities. This helps in the removal of contaminants from wastewater. Matteson et al. (1995) described a device, referred to as an “electronic coagulator” which electrochemically dissolved aluminum (from the anode) into the solution, reacting this with the hydroxyl ion (from the cathode) to form aluminum hydroxide. The aluminum hydroxide, thus formed, flocculates and coagulates the suspended solids and thereby purifies waste water. Carmona et al. (2006) reported that Al or Fe was usually used as electrode material and their actions were generated by the dissolution of sacrificial anodes upon the application of a direct current. This electrolytic process of generating metallic hydroxide flocks in situ via electro-dissolution of the sacrificial anode immersed in the waste water is referred to as electrocoagulation (EC). The generation rate of flocks can be controlled by applying varying amount of current. The electrochemically generated metallic ions can be hydrolyzed next to the anode and generate a series of metal hydroxides that are able to destabilize the dispersed particles present in the wastewater. The destabilized particles are believed to be responsible for the aggregation and precipitation of the suspended particles and for the adsorption of the dissolved and/or colloidal pollutants which are subsequently removed by sedimentation and/or flotation (Bayramoglu et al. 2004; Lung Chou 2010). Thus, the EC process offers the possibility of anodic oxidation which leads to in situ generation of adsorbents such as hydrous ferric oxides, hydroxides of aluminum, etc. (Kumar et al. 2004). The electrode material has a significant effect on the treatment efficiency in terms of cost of the treatment and removal of pollutants, and iron and aluminum electrodes are reasonably inexpensive and are easily available. These electrodes are anodically soluble leading to high wear and tear and thus generate sludge (Mollah et al. 2001; Holt et al. 2002; Kobaya and Can 2003; Daneshvar et al. 2003; Bayramoglu et al. 2004 and Chen 2004).
Electrolytic mechanism with Al and Fe electrodes
The electrolytic process involves the generation of coagulants in situ by electrolytic oxidation of the sacrificial electrode material. Aluminum or iron is usually used as electrodes and their cations are generated by the dissolution of sacrificial anodes upon the application of direct current. The metal ions generated are hydrolyzed in the electrochemical cell to produce metal hydroxide ions according to the reactions (1)–(7) and the solubility of the metal hydroxide complexes formed depends on pH and ionic strength. Insoluble flocs are generated in a pH range between 6.0 and 7.0 as seen from the solubility diagrams of aluminum hydroxide at various pH values (Bensadok et al. 2008). The Al plates are also finding applications in wastewater treatment either alone or in combination with Fe plates due to the high coagulation efficiency of Al3+ (Chen 2004). Mollah et al. (2001) had reported that the electrolytic dissolution of the Al anode produces the cationic monomeric species such as Al3+ and Al(OH)2+ under acidic conditions. At appropriate pH values, they are transformed initially into Al(OH)3 and finally polymerized to Al n (OH)3n according to the following reactions:
However, depending on the pH of the aqueous medium, other ionic species, such as Al(OH)2+, Al2(OH)24+ and Al(OH)4− may also be present in the system.
In addition, various forms of charged multimeric hydroxo Al3+ species may be formed under appropriate conditions. These gelatinous charged hydroxo cationic complexes can effectively remove pollutants by adsorption (Yetilmezsoy et al. 2009).
When a DC electric field is applied, the following electrolysis reactions are expected in the vicinity of the iron electrodes (Ofir et al. 2007).
At anode:
At the cathode:
Overall
Generation of iron hydroxide is followed by an electrophoretic concentration of colloids (usually negatively charged), which are then swept by the electric field in the region close to the anode. The particles subsequently interact with the iron hydroxide and can be removed by the electrostatic attraction. In the region close to the anode, the high concentration of local iron hydroxide increases the probability of coagulation of colloids.
The present investigation was focused on the electrolytic treatment of secondarily treated sewage (STS) water and to find out the removal efficiency of Al–Fe and Fe–Al electrode combinations with different electrode surface areas and inter-electrode distances.
Materials and methods
Collection of wastewater samples
The samples of STS water were collected from the outlet of activated sludge process (ASP) of the sewage treatment plant (STP), Jagjeetpur, Haridwar (Uttarakhand), India, brought to the laboratory and then used for electrolytic treatment using Al–Fe and Fe–Al electrode combinations.
Electrolytic experimental set up
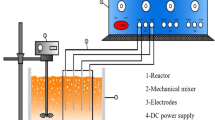
The schematic arrangement of the experimental setup is shown in Fig. 1. The experiments were carried out in a cylindrical reactor having a capacity of 5 L. Al and Fe electrode plates in two combinations (Al–Fe and Fe–Al) having different surface areas (40, 80, 120 and 160 cm2) were connected to the respective anode and cathode leading to the DC rectifier and energized for a required duration of time at a fixed voltage. The inter-electrode distances between the two neighboring electrode plates varied between 2.5 and 10 cm (Table 1). All the experiments were performed at room temperature (30 ± 2 °C) and at a constant stirring speed (100 rpm) to maintain the uniform mixing of effluent during the electrolytic procedure. Before conducting an experiment, the electrodes were washed with water, dipped in dilute hydrochloric acid (HCl, 5 % v/v) for 5 min, thoroughly washed with water and finally rinsed twice with distilled water. After electrolytic treatment, the effluent was allowed to stand for 2 h and then sampled for analysis.
Analytical methods
The TD, COD and BOD of wastewater were analyzed before and after the electrolytic treatment following the standard methods for examination of water and wastewater (APHA 2005). The calculation of TD, COD and BOD removal efficiencies after electrolytic treatment was carried out using the formula:
where C0 and C are TD, COD or BOD of wastewater before and after electrolysis.
Results and discussion
Removal of chemical and biological impurities from the contaminated water system by the process of electrolysis is governed by several factors including electrode material and distance between them, time of electrolysis, electrical parameters such as voltage and current densities, pH of the system and last but not the least the presence of other coagulants in the system, This preliminary study was devoted to figure out effects of electrode systems consisting of different materials on STS water treatment. Changes in surface area and distance between the electrodes were studied in detail. The characteristics of different parameters of the STS water are given in Table 2. The removal of TD, COD and BOD of STS water with electrode combinations (Al–Fe and Fe–Al) using different surface areas (40–160 cm2) and different inter-electrode distances (2.5–10 cm) are shown in Figs. 2, 3, 4, 5 and 6.
Inter-electrode distance was observed to be an effective factor in the electrolytic treatment of STS water. The removal percentage of TD, COD and BOD increased progressively with decrease in inter-electrode distance from 10.0 to 2.5 cm, whereby it exhibited the maximum removal of TD (65.9 %), COD (57.41 %) and BOD (59.56 %) at the shortest distance (2.5 cm) between the electrodes (Al and Fe) with each electrode area of 80 cm2, whereas the Fe and Al electrode combination showed the removal of TD (59.66 %), COD (56.46 %) and BOD (51.99 %) (Figs. 2, 3). Similar observations have also been reported by Li et al. (2008) that COD decreases with the decrease in distance between electrodes of the same composition. This is because the shorter distance speeds up the anion discharge on the anode and improves the oxidation. It also reduces resistance, the electricity consumption and the cost of the wastewater treatment. Ghosh et al. (2008) have also observed that with the increase of inter-electrode distance, the percentage removal of dye products from waste water decreases. At a lower inter-electrode distance, the resistance encountered by current flowing in the solution medium decreases thereby facilitating the electrolytic process and resulting in enhanced dye removal. The above results also indicated the superiority of aluminum as sacrificial electrode when compared to that of iron as sacrificial electrode. This can probably be attributed to better coagulating properties of Al3+ to those of oxidized products of Fe. It may be due to the fact that the majority of Al3+ ions subsequently precipitates in the form of hydroxides. The adsorption of Al3+ ion with colloidal pollutants results in coagulation, and resulting coagulants can be more efficiently removed by settling, surface complexation and electrostatic attraction in comparison to Fe2+ ions.
With a fourfold increase in the electrode area of Al–Fe from 40 to 160 cm2, the current increased from 0.24 to 0.58 A; this resulted in an increase in the removal percentage of TD, COD and BOD. Highest removal efficiencies of 81.51 % (TD), 74.36 % (COD) and 70.86 % (BOD) were achieved at electrode area of 160 and at 2.5 cm inter-electrode distance. The removal efficiency can be attributed to a greater electrode area that produced larger amounts of anions and cations from the anode and cathode. The greater electrode area increased the rate of flock’s formation, which in turn influenced the removal efficiency (Figs. 4, 5). Escobara et al. (2006) have also observed logistical relationship between electrode geometric area (AG) and copper removal efficiency and concluded that the increase in copper removal was related to an increase in AG, reaching an optimal value of 35 cm2, with an asymptotic value near 80 %. In the case of Fe–Al electrode combination the removal efficiency of TD, COD and BOD was 71.11, 64.95 and 61.87 %, respectively, which was somewhat lower than the values obtained with Al–Fe electrode.
Also in the present study, the electrolytic reactor equipped with a higher electrode area of Al and Fe electrode combinations was able to produce significant quantities of coagulants, thereby indicating the enhancement in removal efficiency of TD, COD and BOD from STS water. The increase in the electrode area during electrolytic treatment was predicted to an increase in number of hydroxide ions (OH−) in solution resulting from water reduction at the cathode.
In the electrolytic treatment, the selection of suitable electrode material is important and so is the time required to effect an acceptable removal of dissolved and undissolved impurities. Therefore, we studied the electrolytic treatment using both electrode combinations under the same conditions but as a function of time. The comparative results of TD, COD and BOD removal, obtained with the same voltage (12 V), same inter-electrode spacing (2.5 cm) and same area of electrode (160 cm2) but a varying time of up to 30 min again demonstrated the superiority of Al–Fe electrode combination over that of Fe–Al electrode combination (Fig. 6). During their study of the electrolytic treatment of latex wastewater, Vijayaraghavan et al. (2008) also observed that the increase in the electrolysis period resulted in a decrease in residual COD and BOD concentrations irrespective of the current densities. An increase in the operating time from 10 to 60 min in the treatment of the baker’s yeast wastewater by electrocoagulation resulted in an increase in the removal efficiencies of COD, TOC and turbidity as reported by Kobya and Delipinar (2008).
Metallic hydroxides are produced up to a sufficient concentration of coagulant inducing the formation of white and slightly greenish precipitate using Al–Fe and Fe–Al electrode combination as the OH− of Al and Fe, respectively. This indicates that the STS water can be efficiently treated with Al–Fe combination that ensures better adsorption of soluble and colloidal species which settles down in the form of Al(OH)3 from STS water. Zongo et al. (2009) in their investigation of electro-coagulation for the treatment of textile wastewater with Al or Fe electrode elucidated that the Fe electrode is easily dissolved in water in comparison with Al. However, the use of iron electrodes often results in the formation of very fine brown particles which are less prone to settling than the gel flows formed with aluminum. For further re-use of the treated water, the post-treatment downstream of the electro-coagulation–electro-flotation system might represent a penalty to the use of iron over aluminum.
The present finding is in support of Lai and Lin (2003) who observed that the Al–Fe electrode pair is deemed to be a better choice out of the five electrode pair combinations tested. They also observed that Al–Fe electrode pair offers good overall COD and copper removal, low final wastewater NTU and reasonably low sludge production. Adhoum and Monser (2004) using Al electrodes achieved a COD removal efficiency of 76 % in the treatment of olive mill effluents. Ilhan et al. (2008) indicated the maximum removal of COD 56 and 35 % on the EC of leachate using Al and Fe electrode, respectively, in 30-min contact time. According to Lung Chou (2010), removal efficiency of polyvinyl alcohol (PVA) from aqueous solutions for Fe–Al and Fe–Fe pairs using Fe as the anode was greater than those of Al–Al and Al–Fe pairs using Al as the anode. This has been explained by the chemical reactions that take place at the aluminum anode and the iron anode. Katal and Pahlavanzadeh (2011) observed that the Fe–Al electrode combination has higher COD removal efficiency in comparison to Al–Fe electrode combination, while in present study, Al–Fe electrode combination was more efficient in comparison to Fe–Al electrode combination. In our opinion, this difference can probably be attributed to the different types of contaminants present in the waste water being studied. Iron is a 3d block transition metal and has better complexing properties with organic/inorganic impurities present in water which can act as complexing ligands than aluminum which is a p block metal and lacks empty d-orbitals necessary for making coordination compounds. Therefore, Fe–Al electrode system may be a better choice if electron-rich nucleophilic organic compounds such as dyes, their intermediates and degradation products are present in waste water. However, in the present study, Al–Fe system was found to be more efficient. Therefore, chemical behavior of the contaminants present in waste water may be a deciding factor in the selection of anodic sacrificial electrode.
Energy consumption and operating cost
Electrical energy and electrode consumption are important economical parameters in EC process. In EC process, the operating cost includes material, mainly electrodes and electrical energy costs, as well as labor, maintenance, sludge dewatering and its disposal. In the present study, energy and electrode material costs have been taken into account as major cost items in the calculation of the operating cost (US $/m3) (Ghosh et al. 2008) as follows:
where Cenergy (kWh/m3) and Celectrode (kg Al/m3) are the consumption quantities for the turbidity, COD and BOD removal, “a” is the electrical energy price 0.1 US$/kWh, “b” is the electrode material price 3.4 US$/kg for Al electrode and 1.3 US$/kg for Fe electrode. Cost due to electrical energy (kWh/m3) is calculated as:
Cost for electrode (kg Al/m3) was calculated as follows using the equation:
where U is the cell voltage (V), I current (A), tEC time of electrolysis (s), v volume (m3) of STS water, MW molecular mass of aluminum (26.98 g/mol) and iron (55.84 g/mol), z no. of electrons transferred (z = 3 for Al and 2 for Fe) and F is the Faraday’s constant (96487C/mol) .
For both electrode combinations (Al–Fe and Fe–Al), the energy consumption increased from 1.04 to 2.5 kWh/m3 with an increase in current from 0.24 to 0.58 A that resulted in increasing the electrode consumption (0.85 × 10−5 to 6.04 × 10−5 kg/m3). The cost due to electrical energy consumption as well as an electrode assembly was calculated for both electrode combinations at optimum operating condition. The operating cost of Fe–Al (0.25006 US$/m3) electrode combination was found to be slightly higher than Al–Fe (0.22906 US$/m3) electrode combination (Table 3).
Kinetic study of turbidity, COD and BOD
The rate of removal of TD, COD and BOD is represented by the following first-order mechanism (El-Ashtoukhy and Amin 2010):
where C0 is the initial concentration (mg/L), C t final concentration with respect to time, t the time (min) and k is the rate constant (min−1) for TD, COD and BOD for electrolytic treatment using Al–Fe and Fe–Al electrode combination. Rate constants for electrolytic treatment of TD, COD and BOD from STS water using two types of electrode combination are given in Table 4.
The kinetic study on the distances between electrodes for electrolytic treatment has not been given due consideration so far. There appears to be no work with regard to the kinetic study on the distance between electrodes during electrolytic treatment. In the present study, it was revealed that there is a strong positive correlation between inter-electrode space, and TD, COD as well as BOD abatement rates and rate of coefficients. The pseudo-first-order abatement kinetic was relatively fitted. The decrease in the distance between electrodes from 10.0 to 2.5 cm increased the rate constant from 0.01 to 0.026 min−1 for TD, 0.007 to 0.020 min−1 for COD and 0.006 to 0.018 min−1 for BOD using Al–Fe electrode combination and from 0.008 to 0.019 min−1 for TD, 0.006 to 0.015 min−1 for COD and 0.005 to 0.014 min−1 for BOD using Fe–Al electrode combination. The increase in the rate constant of both Al–Fe and Fe–Al electrode combinations may be ascribed to the decrease of TD, COD and BOD of the STS water. The use of this kinetic study showed high correlation coefficients (r2 ≥ 0.959). Thus, the kinetic study is more suitable for explaining the efficiency of distance between electrodes for electrolytic treatment.
Conclusion
The use of electrode systems using different metals for anodes and cathodes was studied in an attempt to improve upon the existing system and to further understand the process of electrolysis. The removal of TD, COD and BOD was found to be dependent on the inter-electrode distances, electrode areas and the electrode combinations (Al–Fe and Fe–Al) in the treatment of STS water. An increase in the surface area of the electrodes and a decrease in the distance between them resulted in better removal of contaminants from the waste water; the optimal removal has been obtained with the use of an electrode area of 160 cm2 and a short distance of 2.5 cm between electrodes in a 5-L reactor. Acquired results of the present study could be specified and evaluated by employing pseudo-first-order kinetics. The electrical energy consumption was calculated as 0.229 kWh/m3 for Al–Fe and 0.25 kWh/m3 for Fe–Al electrode combination. Al–Fe electrode combination proved more effective in comparison to Fe–Al electrode combination for the treatment of STS water. Due to economical constraint, EC with Al–Fe electrode combination should be preferred in comparison to Fe–Al electrode combination. Al anode was more efficient in comparison to Fe anode establishing the superiority of aluminum as the preferred material for sacrificial electrode for the treatment of sewage water obtained from STP, Jagjeetpur, Haridwar (Uttarakhand), India.
References
Adhoum N, Monser L (2004) Decolourization and removal of phenolic compounds from olive mill wastewater by electro coagulation. Chem Eng Process 43:1281–1287
APHA (2005) Standard methods for the examination of water and wastewater, American Public Health Association, 21st edn. Washington, DC
Bayramoglu M, Kobya M, Can OT, Sozbir M (2004) Operating costs analysis of electrocoagulation of textile dye wastewater. Sep Purif Technol 37:117–125
Bensadok K, Benammar S, Lapicque F, Nezzal G (2008) Electrocoagulation of cutting oil emulsions using aluminum plate electrodes. J Hazard Mater 152(1):423–430
Carmona M, Khemis M, Leclerc JP, Lapicque F (2006) A simple model to predict the removal of oil suspensions from water using the electrocoagulation technique. Chem Eng Sci 61:1237–1246
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38:11–41
Comninellis Ch (1994) Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for wastewater treatment. Electrochim Acta 39(11):1857–1862
Daneshvar N, Ashassi-Sorkhabi H, Tizpar A (2003) Decolorization of orange II by electrocoagulation meted. Sep Purif Technol 31:153–162
El-Ashtoukhy ESZ, Amin NK (2010) Removal of acid green dye 50 from wastewater by anodic oxidation and electrocoagulation—a comparative study. J Hazard Mater 179:113–119
Escobara C, Cesar SS, Toral M (2006) Optimization of the electrocoagulation process for the removal of copper, lead and cadmium in natural waters and simulated wastewater. J Environ Manag 81(4):384–391
Ghosh D, Medhi CR, Solanki H, Purkait MK (2008) Decolorization of crystal violet solution by electrocoagulation. J Environ Prot Sci 2:25–35
Holt PK, Barton GW, Wark M, Mitchell CA (2002) A quantitative comparison between chemical dosing and electrocoagulation. Colloids Surf A Physicochem Eng Aspects 211:233–248
Ilhan F, Kurt U, Apaydin O, Gonullu MT (2008) Treatment of leachate by electrocoagulation using aluminum and iron electrodes. J Hazard Mater 154:381–389
Katal R, Pahlavanzadeh H (2011) Influence of different combinations of aluminum and iron electrode on electrocoagulation efficiency: Application to the treatment of paper mill wastewater. Desalination 265:199–205
Kobaya MOT, Can Bayramoglu M (2003) Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. J Hazard Mater B 100:163–178
Kobya M, Delipinar S (2008) Treatment of the baker’s yeast wastewater by electrocoagulation. J Hazard Mater 154:1133–1140
Kumar PR, Chaudhari S, Khilar KC, Mahajan SP (2004) Removal arsenic from water by electrocoagulation. Chemosphere 55:1245–1252
Lai CL, Lin SH (2003) Electrocoagulation of chemical mechanical polishing (CMP) wastewater from semiconductor fabrication. Chem Eng J 95:205–211
Li Xu, Wang Wei, Wang Mingyu, Cai Yongyi (2008) Electrochemical degradation of tridecane dicarboxylic acid wastewater with tantalum-based diamond film electrode. Desalination 222:388–393
Lung Chou W (2010) Removal and adsorption characteristics of polyvinyl alcohol from aqueous solutions using electrocoagulation. J Hazard Mater 177:842–850
Matteson MJ, Dobson RL, Glenn RW Jr, Kukunoor NS, Waits WH III, Clayfield EJ (1995) Electrocoagulation and separation of aqueous suspensions of ultrafine particles. Colloids Surf A 104(1):101–109
Mollah MYA, Schennach R, Parga JR, Cocke DL (2001) Electrocoagulation (EC)—science and applications. J Hazard Mater B 84:29–41
Ofir E, Oren Y, Adin A (2007) Comparing pretreatment by iron of electro flocculation and chemical flocculation. Desalination 204:87–93
Simonsson D (1997) Electrochemistry for a cleaner environment. Chem Soc Rev 26:181–189
Vijayaraghavan K, Ahmad D, Yuzri A, Yazid A (2008) Electrolytic treatment of latex wastewater. Desalination 219:214–221
Yetilmezsoy K, Ilhan F, Zengin ZS, Sakar S, Gonullu MT (2009) Decolorization and COD reduction of UASB pretreated poultry manure wastewater by electrocoagulation process: A post-treatment study. J Hazard Mater 162:120–132
Zongo I, Maiga AH, Wéthé J, Valentina G, Leclerca JP, Paternottea G, Lapicquea F (2009) Electrocoagulation for the treatment of textile wastewaters with Al or Fe electrodes: compared variations in COD levels, turbidity and absorbance. J Hazard Mater 169:70
Acknowledgments
The University Grant Commission, New Delhi, India is acknowledged for providing the financial support in the form of UGC research fellowship (F.4-1/2006 (BSR) 7-70/2007 BSR) to Mr. Arun Kumar Sharma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chopra, A.K., Sharma, A.K. Removal of turbidity, COD and BOD from secondarily treated sewage water by electrolytic treatment. Appl Water Sci 3, 125–132 (2013). https://doi.org/10.1007/s13201-012-0066-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-012-0066-x