Abstract
The present study envisages the importance of graphical representations like Piper trilinear diagram and Chadha’s plot, respectively to determine variation in hydrochemical facies and understand the evolution of hydrochemical processes in the Varahi river basin. The analytical values obtained from the groundwater samples when plotted on Piper’s and Chadha’s plots revealed that the alkaline earth metals (Ca2+, Mg2+) are significantly dominant over the alkalis (Na+, K+), and the strong acidic anions (Cl−, SO4 2−) dominant over the weak acidic anions (CO3 2−, HCO3 −). Further, Piper trilinear diagram classified 93.48 % of the samples from the study area under Ca2+–Mg2+–Cl−–SO4 2− type and only 6.52 % samples under Ca2+–Mg2+–HCO3 − type. Interestingly, Chadha’s plot also demonstrated the dominance of reverse ion exchange water having permanent hardness (viz., Ca–Mg–Cl type) in majority of the samples over recharging water with temporary hardness (i.e., Ca–Mg–HCO3 type). Thus, evaluation of hydrochemical facies from both the plots highlighted the contribution from the reverse ion exchange processes in controlling geochemistry of groundwater in the study area. Further, PCA analysis yielded four principal components (PC1, PC2, PC3 and PC4) with higher eigen values of 1.0 or more, accounting for 65.55, 10.17, 6.88 and 6.52 % of the total variance, respectively. Consequently, majority of the physico-chemical parameters (87.5 %) loaded under PC1 and PC2 were having strong positive loading (>0.75) and these are mainly responsible for regulating the hydrochemistry of groundwater in the study area.
Similar content being viewed by others
Introduction
Groundwater is being used for domestic, agricultural and industrial purposes across the world since time immemorial. As a natural resource, groundwater is required for reliable and commercial delivery of potable water supply in both urban and rural environment for the well-being of humans, some aquatic and terrestrial ecosystems. Recently, water demand has increased rapidly with the construction of energy, development of industry, agriculture, urbanization, improvements in living standards and eco-environment construction. Evaluation of quality and suitability of groundwater for various utilitarian purposes are acquainting extra concern in the present day life. Thus, investigations associated with understanding of the hydrochemical characteristics of the groundwater, geochemical processes involved and its evolution under natural water circulation processes not only helps in effective utilization and protection of this valuable resource but also aid in envisaging the alterations in groundwater environment (Lawrence et al. 2000; Edmunds et al. 2006). The formation of various hydrochemical facies/water types will be influenced by geochemistry of groundwater present within an aquifer, which is further regulated by interaction between composition of the precipitation, geological structure, mineralogy of the watersheds/aquifers and the geological processes within the aquifer. The general chemical nature of ground water and variation in hydrochemical facies can be understood by plotting major cation and anion concentrations on different graphical representations. Hence, an attempt was made in the present study to demonstrate the variation in hydrogeochemistry in Varahi river basin using Piper trilinear diagram and Chadha’s plots. Further, statistical aid such as principal component analysis (PCA) was used to find out the major contributing parameters involved in deciding the geochemistry of groundwater samples.
Study area
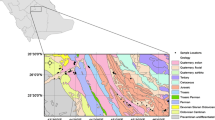
River Varahi is a major west flowing river on the west coast in Udupi district, which originates from the high peaks of the Western Ghats near a Guddakoppa village in Hosanagar taluk, Shimoga district at an altitude of about 761 m above MSL and flows for a length of 88 kms. The Varahi irrigation project site is located at approximately 6 kms from Siddapura, Kundapura taluk, Udupi district with a latitude of 13°-39′15″N and a longitude of 74°-57′0″E with a total drainage area of the river is about 755.20 sq km. The stream collects heavy rainfall in the hilly region around Agumbe and Hulikal. Tributaries like Hungedhole, Kabbenahole, Dasnakatte, Chakranadi, etc., will join Varahi before emptying into the Arabian Sea. The study area is having a catchment area of 293.0 km2 (29,300 ha) command area of 157.02 km2 (15,702 ha) covering part of Kundapura (83.24 km2) and Udupi (73.78 km2) taluks of Udupi District (Fig. 1). The reservoir water has been directed by via Varahi Left Bank Canal (VLBC, 33 km) and Varahi Right Bank Canal (VRBC, 44.70 km) to irrigate an area of around 27.23 km2 (2723 ha) and 19.92 km2 (1992 ha), respectively. The net irrigable command area is 129.79 km2 (12,979 ha) by flow irrigation and correspondingly 27.23 km2 (2723 ha) by lift irrigation, to provide enhanced irrigation facilities and an improved drinking water system to the villages of two taluks of Udupi district by means of the canal system. The Varahi river basin fall under coastal zone of the tenfold agro-climatic zone of Karnataka, with moderately hot climate and enjoys a pleasant temperature range from the highest mean maximum of 35 °C to lowest mean maximum of 23 °C with a mean temperature of 27 °C. South Canara is a thickly populated area in general and Udupi district in particular, which receives plenty of rainfall during South West Monsoon. The mean annual rainfall is 539.97 cm (5399.68 mm) with a maximum of 632 cm (6320 mm) and a minimum of 318 cm (3180 mm), while the mean relative humidity is 76 %.
Geology and hydrogeology
Varahi River basin comprises of varying slopes such as gentle, moderate, moderately steep, nearly level, strong slope, very gentle and very steep, with slope values varying from 0–35 %. Topology of the region is generally flat with nearly level slope with its value varying between 0 and 1 % and the area lies between 25 and 40 m above mean sea level. The geomorphology of the region is generally plain and piedmont zone with patches of hills, plateaus here and there. The soil of the Varahi River basin comprises of Clayey skeletal followed by patches of sandy loam and loamy sand. Cape comorin to Sharavati basin covers major portion of Udupi district, characterized by Netravati to Sita and Sita to Sharavati subcatchments. Udupi district is characterized by various geological formations belonging mainly to Archean and Upper proterozoic/palaeocene to recent periods. Geologically, peninsular gneisses cover the Varahi river basin area, mainly contains Hornblende-Biotite Gneiss, Hornblende Granite and patches of Laterite. The metamorphic and plutonic rock types cover major portion of the district with patches of residual capping and unconsolidated sediments (Fig. 1). The groundwater occurs in the weathered mantle of the granitic gneisses and joints, cracks and crevices of basement rocks. The weaker parts like lineaments and joints with an orientation towards the NNE–SSW are prominent in study area, partly responsible for controlling the groundwater flow in the study area.
Methodology
For hydrochemical analyses (viz., major anions and cations), 46 groundwater samples spread over Varahi river basin were collected in polyethylene bottles during March 2010 (pre-monsoon season). The sampling bottles were soaked in 1:1 diluted HCl solution and washed twice with distilled water before sampling and were washed again in the field with groundwater sample filtrates. Clear pumping for 10 min was carried out until stable meter readings of the in-situ parameters (temperature, pH, Eh and EC) was obtained using portable digital meters. This was done to avoid the sampling of stagnant annulus water that would be in the region of the pump and pump systems and to prevent changes in the chemistry of the water samples before analyses. For analysis of major cations, samples were filtered through 0.45 μm cellulose acetate filter membrane using filtering apparatus and then by adding ultra-pure HNO3 in the field until the pH is ≤2. Samples were also stored separately at 4 °C without preservation for major anions. The preservation of samples in the field, their transportation and analysis of major ions in the laboratory has been carried out using the standard recommended analytical methods (APHA 2005). All values are reported in milligram per liter, unless otherwise indicated.
Piper trilinear diagrams were plotted using Aquachem 3.7 software package while MS Excel spreadsheet was used to create the Chadha’s diagram. Further, the analytical results shown in Table 1 were used as input for principal component (PCA) analysis. The extraction methods of Varimax rotation and Kaiser normalization were applied to interpret the geochemical data using IBM SPSS Statistics v20 and Minitab v15 software.
Results and discussion
The chemical compositions of the groundwater samples are shown in Table 1 and as box plot in Fig. 2. In Varahi river basin, average pH was 6.64, with a maximum and minimum of 8.27 and 4.57, indicating that moderately acidic to slightly alkaline nature of water samples and 43.48 % of the samples showed the pH value exceeding the BIS permissible limit of 6.5–8.5 (BIS 1998).
Electrical conductivity (EC) is a measure of an ability to conduct current and higher EC indicates the enrichment of salts/dissolved matter in the groundwater. The EC values ranged from 29.3 to 1775 μS/cm, with a mean of 163.64 μS/cm. The water can be classified based on EC as type I, if the enrichments of salts are low (EC: <1500 μS/cm); type II, if the enrichment of salts are medium (EC: 1500 and 3000 μS/cm); and type III, if the enrichments of salts are high (EC: >3000 μS/cm) (Subba Rao et al. 2012). Accordingly, all the groundwater samples fall under type I (low enrichment of salts) in the study area except for one sample, as the latter belongs to medium salt enrichment class (type II).
Total dissolved solids ranged from 18.17 to 1100.5 mg/L, and with an average of 101.45 mg/L. Water can be classified based on TDS values (USSL 1954) into freshwater (<1000 mg/L), brackish water (1000–10,000), saline water (10,000–100,000) and brine water (>100,000). All the samples except one sample fall freshwater category. None of the samples showed conductivity and TDS value exceeding their permissible limit of 3000 μS/cm and 2000 mg/L (BIS 1998).
The salinity values varied from 0.01 to 0.89 ‰ (average: 0.08 ‰) while the Eh values varied from −65.3 to 150.8 mV (mean: 31.74 mV). The temperature of water samples varied from 26° to 33 °C, with a mean value of 28.42 °C. The total alkalinity (as CaCO3) was in the range of 13.4–266.7 mg/L (average: 36.94 mg/L), well within the permissible limit of 600 mg/L (BIS 1998). Average total hardness (as CaCO3) was 63.30 mg/L, with a maximum and minimum value of 20–436 mg/L, well below the permissible limit of 600 mg/L (BIS 1998). All samples in Varahi river basin showed the hardness value higher than the alkalinity value illustrating non-carbonate hardness. The calcium hardness (as CaCO3) values ranged from 10 to 200 mg/L (mean: 35.22 mg/L), within the permissible limit of 200 mg/L (BIS 1998).
Cation chemistry (Ca2+, Mg2+, Na+, K+)
Calcium was dominant among all the cations, suggesting that 65.22 % of the samples were calcium-rich water (Fig. 3a) and remaining 34.78 % plotted near the central zone have no dominant cation. Among the alkaline earths, the concentration of Ca and Mg ions ranged from 4 to 80 and 2.44 to 57.58 mg/L, with a mean of 14.09 and 6.85 mg/L, respectively. Among alkalies, the concentration of Na and K ions ranged from 0.8 to 7.5 and 0.1 to 24.4 mg/L, respectively. None of the samples showed the calcium, magnesium and sodium concentration above their respective permissible limit of 200, 100 and 100 mg/L (BIS 1998), but only 2.17 % of the samples showed potassium value above the permissible limit of 10 mg/L (BIS 1998). Their concentrations (on the basis of mg/L) represent on an average, 54.43, 26.48, 12.23 and 6.86 % of all the cations, respectively. The order of abundance of major cations was Ca > Mg > Na > K.
Anion chemistry (HCO3 −, Cl−, SO4 2−, F−, PO4 3−, NO3 −)
For the anions, the concentrations of HCO3, Cl, SO4, NO3, F and PO4 fall between 16.4 and 325.4, 6.0 and 222.5, 3.33 and 134.5, 0.3 and 6.0, 0.04 and 0.45, 0.1 and 0.35 mg/L, respectively, with a mean of 45.07, 22.60, 18.93, 1.40, 0.19 and 0.18 mg/L. The abundance of major ions in groundwater was in the order of HCO3 > Cl > SO4 > NO3 > F > PO4 for anions, and they contribute on an average of 51.01, 25.57, 21.42, 1.58, 0.21 and 0.20 %, respectively, to the total anion content. More than half of the samples are categorized as bicarbonate waters as bicarbonate was the dominant anion (Fig. 3b) and in some samples falling near the central zone have no dominant anion. None of the samples showed a concentration of Cl, SO4, NO3 and F above their respective permissible limit of 1000, 400, 45 and 1.5 mg/L (BIS 1998; WHO 2004). Only one sample showed PO4 concentration above its BIS permissible limit of 0.3 mg/L.
Hydrochemical facies
Graphical representation of groundwater major dissolved constituents (major cations and major anions) helps in understanding its hydrochemical evolution, grouping and areal distribution. In the present study, Piper trilinear diagram and Chadha’s plot were constructed to evaluate variation in hydrochemical facies.
Piper trilinear diagram
It is evident from piper plot (Piper 1944) that out of 46 samples, 93.48 % of the samples belong to Ca2+–Mg2+–Cl−–SO4 2− type and only 6.52 % samples fall under Ca2+–Mg2+–HCO3 − type, illustrating the presence of both permanent and temporary hardness in the groundwater of the Varahi river basin (Fig. 4; Table 2). The graphs also demonstrate the dominance of alkaline earths over alkali (viz., Ca + Mg > Na + K), and strong acidic anions exceed weak acidic anions (i.e., Cl + SO4 > HCO3). It is also observed from Fig. 4 that most of the groundwater samples (76.09 %) are in zone 9, the mixed zone, where types of groundwater cannot be identified as neither anion nor cation dominant (Todd and Mays 2005). While those falling under zone 6 (21.74 %) belong to the permanent hardness category and exhibited calcium chloride type wherein non-carbonate hardness exceeds 50 %, giving an indication of groundwater from formations that are composed of limestone and dolomite or from active recharge zones with short residence time (Hounslow 1995). Remaining 2.17 % samples in zone 5 belong to the temporary hardness class and exhibited magnesium bicarbonate type having carbonate hardness over 50 %, illustrating reverse/inverse ion exchange (Davis and Dewiest 1966) responsible for controlling the chemistry of the groundwater. None of the samples fall under zone 7 and 8 and hence water types originating from halite dissolution (saline) or alkali carbonate enrichment are absent.
Chadha’s plot
The geology, environment and movement of water control the type and concentration of salts in natural waters (Raghunath 1982; Gopinath and Seralthan 2006). Hence, a hydrochemical diagram proposed by Chadha (1999) was also applied to identify different hydrochemical processes. The data was converted to percentage reaction values (milliequivalent percentages), and expressed as the difference between alkaline earths (Ca + Mg) and alkali metals (Na + K) for cations, and the difference between weak acidic anions (HCO3 + CO3) and strong acidic anions (Cl + SO4). The hydrochemical processes suggested by (Chadha 1999) are indicated in each of the four quadrants of the graph. These are broadly brief as reverse ion exchange water (Ca–Mg–Cl type), Recharging water (Ca–Mg–HCO3 − type), seawater/end-member waters (NaCl type), and base ion exchange water (Na–HCO3 type). Recharging waters are formed when water enters into the ground from the surface, it carries dissolved carbonate in the form of HCO3 and the geochemically mobile Ca. Reverse ion exchange waters are less easily defined and less common, but represent groundwater where Ca + Mg is in excess to Na + K either due to the preferential release of Ca and Mg from mineral weathering of exposed bedrock or possibly reverse base cation exchange reactions of Ca + Mg into solution and subsequent adsorption of Na onto mineral surfaces. Seawater types are mostly constrained to the coastal areas as they show typical seawater mixing. Finally, base ion exchange waters, which are more prominent in the study area form a wide band between the western part of the study area and the sea coast and possibly represent base exchange reactions or an evolutionary path of groundwater from Ca-HCO3-type fresh water to Na–Cl mixed seawater where Na-HCO3 is produced by ion exchange processes.
The positions of data points at field 6 (Ca–Mg–Cl type, Ca–Mg dominant Cl type or Cl dominant Ca–Mg type waters) exhibited in Fig. 5 signifies the predominance of reverse ion exchange in majority of the samples; such water will have a permanent hardness and does not deposit residual sodium carbonate in irrigation use and hence, foaming problem will not arise. In contrast, recharge characteristics were observed in very less samples falling in field 5 (recharging waters: Ca–Mg–HCO3 type, Ca–Mg dominant HCO3 type or HCO3 dominant Ca–Mg type waters), having temporary hardness. While no representation of samples either in field 7 (seawater: Na–Cl type, Na dominant Cl type or Cl dominant Na type waters) or 8 (base ion exchange waters: Na–HCO3 type, Na dominant HCO3 type or HCO3 dominant Na type waters), respectively indicating the absence of typical seawater mixing (viz., salinity) or base ion exchange processes (i.e., residual sodium carbonate disposition) in the study area.
Further, Chadha’s (1999) plot can be classified into eight fields as given below: (1) alkaline earths exceed alkali metals, (2) alkali metals exceed alkaline earths (3) weak acidic anions exceed strong acidic anions, (4) strong acidic anions exceed weak acidic anions, (5) alkaline earths and weak acidic anions exceed both alkali metals and strong acidic anions, respectively, (6) alkaline earths exceed alkali metals and strong acidic anions exceed weak acidic anions, (7) alkali metals exceed alkaline earths and strong acidic anions exceed weak acidic anions, and (8) alkali metals exceed alkaline earths and weak acidic anions exceed strong acidic anions. The output of Chadha’s plot (Fig. 5) is in confirmation with that of Piper trilinear diagram (Fig. 4) in that alkaline earths exceed alkali metals (field 1), strong acidic anions exceed weak acidic anions (field 4) and alkaline earths exceed alkali metals and strong acidic anions exceed weak acidic anions (field 6). Only few samples under field 5 specified that alkaline earths and weak acidic anions, respectively, exceed both alkali metals and strong acidic anions as indicated by Ca–Mg–HCO3 type of water.
Principal component analysis
PCA is a powerful technique for pattern recognition that attempts to explain the variance of a large set of inter-correlated variables. It indicates association between variables, thus, reducing the dimensionality of the dataset. PCA extracts the eigen values and eigenvectors from the covariance matrix of original variables. The principal components (PCs) are the uncorrelated (orthogonal) variables, obtained by multiplying the original correlated variables with the eigenvector (loadings). The eigen values of the PCs are the measure of their associated variance, the participation of the original variables in the PCs is given by the loadings, and the individual transformed observations are called scores (Helena et al. 2000; Wunderlin et al. 2001; Singh et al. 2004). The Bartlett’s sphericity test on the correlation matrix of variables demonstrates the calculated χ 2 = 2368.7, greater than the critical value of χ 2 = 59.30 (p = 0.0005 and 28 degrees of freedom), thereby indicating that PCA can accomplish a momentous reduction of the dimensionality of the original dataset. PCA was executed on the correlation matrix of the water dataset, with the intention of identifying a reduced set of factors that could capture the variance of a dataset. Following the criteria of Cattell and Jaspers (1967), PCs with eigen values >1 were retained. Table 3 summarizes the PCA results including the loadings, eigen values and variance elucidated by each principal component (PCs). PCA rendered four PCs with eigen values >1 explaining 89.11 % of the total variance of the dataset.
The scree plot shown in Fig. 6 is the way of identifying a number of useful factors, wherein, a sharp break in sizes of eigen values which results in a change in the slope of the plot from steep to shallow could be observed. The slope of the plot changes from steep to shallow after the first two factors. The eigen values also drop below 1, when we move factor 4 to factor 5. This suggests that a four component solution could be the right choice which includes the total variance of 89.11 %.
The loadings plot (Fig. 7) of the first two PCs (PC1 and PC2) shows the distribution of all the physico-chemical parameters in the first (upper right) and fourth (lower right) quadrants. The lines joining the variables and passing through the origin in the plot of the factor loadings are indicative of the contribution of the variables to the samples. Proximity of lines for two variables signifies the strength of their reciprocal association (Qu and Kelderman 2001). Grouping of parameters (CaH, Ca and SO4; EC, TDS, and TH; K, HCO3 and TA; Cl and Mg; Na and NO3 −) in the loadings plot suggests their significant mutual positive correlation. The PCs score plots portray the characteristics of the samples and aid to comprehend their spatial distribution. The PCs scores plot (Fig. 8) constructed using PC1 and PC2 components confirms the clustering of site specific samples in space and their spatial distribution.
From Figs. 7 and 8, it is apparent that samples distributed in upper quadrants are more concentrated with pH, F, Na, NO3, CaH, Ca, SO4, EC, TDS while, those in the lower quadrants with PO4, TH, K, TA, HCO3, Mg and Cl. The scores plot (PC1 and PC2) for the water samples from Varahi river basin (Fig. 8) shows mixed distribution of samples. Visible grouping of majority of the samples has been observed in the upper left and lower left quadrants while, loading of samples in the upper right and lower right quadrants are found to be noticeably scattered. Finally, it was concluded that PCA analysis yielded four principal components of which, PC1 and PC2 accounting for 65.55 and 10.17 % of total variance, respectively, are mainly responsible for controlling geochemistry of groundwater. This is because 87.5 % of physico-chemical parameters analyzed in the study area and have strong loading (>0.75) fall under these two components. In contrast, PC3 and PC4 exhibited strong and positive loading by one parameter each with an insignificant percentage of variance (i.e., 6.88 and 6.52 %) and were given least preference in regulating groundwater chemistry.
Relationship between EC, SAR and percent sodium
Many water quality parameters affect crop growth and productivity and the suitability of the groundwater for irrigation depends on the mineralization of water and its effect on plants and soil. Suitability of surface irrigation for cultivation can be evaluated using parameters such as conductivity, Na, Ca, Mg, Cl, HCO3, SO4, Percent sodium, RSC, SAR, etc., (Ravikumar et al. 2011; Ravikumar and Somashekar 2011; Ravikumar and Somashekar 2013) of which, total Na+ concentration and EC is considered to be very important in classifying the irrigation water (Raghunath 1987). When the concentration of sodium is high in irrigation water, sodium ions tend to be absorbed by clay particles, displacing Mg2+ and Ca2+ ions. This exchange process of Na+ in water for Ca2+ and Mg2+ in the soil reduces the permeability and eventually results in soil with poor internal drainage. Hence, air and water circulation is restricted during wet conditions and such soils become usually hard when dried (Saleh et al. 1999). Similarly, salinization eventually makes groundwater inadequate for the growth and productivity of many crops (El Moujabber et al. 2006) as the salinity is effective on growth and yield of plants through increasing osmotic pressure and concentration of specific ions. The amount of a particular plant’s tolerance of salinity at different stages is different and for appropriate growth and productivity of most crops, EC (an indirect indicator of salinity) levels should be less than 2000 uS/cm. Soil salinity is the better criterion for evaluating crop growth, but salinity of irrigation water was used in this case because it is easier to measure. Salinity value was within the permissible limit (i.e., 29.3 ≥ EC 1775 μS/cm). Samples were excellent to good type for irrigation based on percent sodium (viz., 1.19 ≥ % Na ≤ 35.28) and SAR (viz., 0.05 ≥ SAR ≤ 0.55). Thus, majority of the samples in the Varahi river basin belong to low/medium sodium and low salinity hazard category (Fig. 9).
Further, there is a tendency for calcium and magnesium to precipitate as the water in the soil becomes more concentrated when bicarbonates are present in higher concentration. Excess quantity of sodium bicarbonate and carbonate (expressed as RSC) causes dissolution of organic matter in the soil, which in turn leaves a black stain on the soil surface on drying, which is detrimental to the physical properties of soils. In the present case, all the samples were considered good for irrigation based on RSC values (<1.25 meq/L) and RSC values were positive in 67.39 % of the samples, suggesting that HCO3 − content is higher than dissolved Ca2+ and Mg2+ ions in water.
Conclusion
The present study demonstrated the importance of constructing graphical representations such as Piper trilinear diagram and Chadha’s plot using dissolved constituents (major cations and major anions) to effectively understand hydrochemical evolution, grouping and areal distribution of water facies of groundwater resources in an area. The Piper trilinear diagram can be used to illustrate the similarities and differences in the composition of waters and to classify them into certain chemical types, while Chadha’s plot can demonstrate the hydrochemical processes like recharging, reverse ion exchange, seawater mixing and base ion exchange chiefly acting in an aquifer. In the present study, piper trilinear diagram classified 93.48 % of the samples under Ca2+–Mg2+–Cl−–SO4 2− type indicating the permanent hardness and remaining 6.52 % samples under Ca2+–Mg2+–HCO3 − type demonstrating temporary hardness. The piper plots further confirmed the existence of mixed type of water with no one cation–anion pair exceeding 50 % in majority of the analyzed samples. Chadha’s plot also demonstrated the dominance of reverse ion exchange water having permanent hardness (viz., Ca–Mg–Cl type) in majority of the samples over recharging water with temporary hardness (i.e., Ca–Mg–HCO3 type). It is therefore evident that primary salinity and secondary alkalinity were dominant in majority of samples as indicated by permanent (non-carbonate) hardness. In contrast, secondary salinity and primary alkalinity were limited to only few samples as specified by temporary (carbonate) hardness. No typical seawater mixing or base ion exchange was observed in the study area as none of the samples belong to Na–Cl and Na–HCO3 water types. Both the plots highlighted the contribution from the reverse ion-exchange processes besides the dominance of alkaline earth metals (Ca2+, Mg2+) over the alkalis (Na+, K+), and strong acidic anions (Cl−, SO4 2−) over the weak acidic anions (CO3 2−, HCO3 −) in the study area. Further, PCA analysis established that water quality parameters under PC1 and PC2 (viz., 65.55 and 10.17 % of total variance) having strong loading (>0.75) was considered to govern the groundwater quality in the study area. While water quality parameters under PC3 and PC4 though exhibited strong and positive loading by one parameter each, were considered insignificant due to a lesser percentage of variance (i.e., 6.88 and 6.52 %). Overall the groundwater quality was suitable for drinking and domestic purposes and permissible for irrigation activities.
References
APHA American Public Health Association (2005) Standard method for examination of water and wastewater, 21st edn. APHA, AWWA, WPCF, Washington
BIS (1998) Drinking water specifications (revised 2003). Bureau of Indian Standards, IS:10500
Cattell RB, Jaspers JA (1967) A general plasmode for factor analytic exercises and research. Multivar Behav Res Monogr 3:1–212
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7(5):431–439
Davis SN, Dewiest RJM (1966) Hydrogeology. John Wiley and Sons Inc., New York, USA, p 463
Edmunds WM, Ma JZ, Aeschbach-Hertig W, Kipfer R, Darbyshire DPF (2006) Groundwater recharge history and hydrogeochemical evolution in the Minqin Basin, North West China. Appl Geochem 21:2148–2170
El Moujabber M, Bou Samra B, Darwish T, Atallah T (2006) Comparison of different indicators for groundwater contamination by seawater intrusion on the Lebanese coast. Water Resour Manage 20:161–180
Gopinath G, Seralthan P (2006) Chemistry of groundwater in the lateritic formation of Muvatterpuzha river basin, Kerala. J Geol Soc India 68:705–714
Helena B, Pardo R, Vega M, Barrado E, Fernandez JM, Fernandez L (2000) Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga river, Spain) by principal component analysis. Wat Res 34:807–816
Hounslow AW (1995) Water quality data: analysis and interpretation. CRC Lewis Publisher, New York, USA, p 396
Lawrence AR, Gooddy DC, Kanatharana P, Meesilp M, Ramnarong V (2000) Groundwater evolution beneath Hat Yai, a rapidly developing city in Thailand. Hydrol J 8:564–575
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analysis. Am Geoph Union Trans 25:914–923
Qu W, Kelderman P (2001) Heavy metal contents in the Delft canal sediments and suspended solids of the River Rhine: multivariate analysis for source tracing. Chemosphere 45(6–7):919–925
Raghunath HM (1982) Groundwater. Wiley, New Delhi, p 456
Raghunath HM (1987) Groundwater, 2nd edn. Wiley Eastern Ltd, New Delhi
Ravikumar P, Somashekar RK (2011) Geochemistry of groundwater, Markandeya River Basin, Belgaum district, Karnataka State, India. Chin J Geochem 30(1):51–74. doi:10.1007/s11631-011-0486-6
Ravikumar P, Somashekar RK (2013) A geochemical assessment of coastal groundwater quality in the Varahi river basin, Udupi District, Karnataka State, India. Arabian J Geosci 6(6):1855–1870. doi:10.1007/s12517-011-0470-9
Ravikumar P, Somashekar RK, Angami M (2011) Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin, Belgaum District, Karnataka State, India. Environ Monitor Assess 173(1):459–487. doi:10.1007/s10661-010-1399-2
Saleh A, Al-Ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—a case study. Water Res 38:3980–3992
Subba Rao N, Surya Rao P, Venktram Reddy G, Nagamani M, Vidyasagar G, Satyanarayana NLVV (2012) Chemical characteristics of groundwater and assessment of groundwater quality in Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ Monit Assess 184:5189–5214. doi:10.1007/s10661-011-2333-y
Todd DK, Mays LW (2005) Groundwater hydrology. Wiley, New York, p 636
USSL (1954) Diagnosis and improvement of saline and alkali soils. USDA Handbook 60:147
WHO (2004) Guidelines for drinking-water quality volume 1: recommendations, 3rd edn. WHO, Geneva
Wunderlin DA, Diaz MDP, Ame MV, Pesce SF, Hued AC, Bistoni MD (2001) Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquia River Basin (Cordoba Argentina). Water Res 35:2881–2894
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ravikumar, P., Somashekar, R.K. Principal component analysis and hydrochemical facies characterization to evaluate groundwater quality in Varahi river basin, Karnataka state, India. Appl Water Sci 7, 745–755 (2017). https://doi.org/10.1007/s13201-015-0287-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0287-x