Abstract
The present study on geochemical evolution of groundwater is taken up to assess the controlling processes of water chemistry in the Western Delta region of the River Godavari (Andhra Pradesh), which is one of the major rice-producing centers in India. The study region is underlain by coarse sand with black clay (buried channels), black silty clay of recent origin (floodplain) and gray/white fine sand of modern beach sediment of marine source (coastal zone), including brown silty clay with fine sand (paleo-beach ridges). Groundwater is mostly brackish and very hard. It is characterized by Na+ > Mg2+ > Ca2+:HCO3 − > Cl− > SO4 2− > NO3 −, Na+ > Mg2+ > Ca2+:Cl− > HCO3 − > SO4 2−, and Mg2+ > Na+ > Ca2+ > or < K+:HCO3 − > Cl− > or > SO4 2− facies. The ionic relations (Ca2+ + Mg2+:HCO3 −, Ca2+ + Mg2+:SO4 2− + HCO3 −, Na+ + K+:TC, Na+ + K+:Cl− + SO4 2−, HCO3 −:TC, HCO3 −:Ca2+ + Mg2+, Na+:Cl− and Na+:Ca2+) indicate that the rock weathering, mineral dissolution, evaporation and ion exchange are the processes to control the aquifer chemistry. Anthropogenic and marine sources are also the supplementary factors for brackish water quality. These observations are further supported by Gibbs mechanisms that control the water chemistry. Thus, the study suggests that the initial quality of groundwater of geogenic origin has been subsequently modified by the influences of anthropogenic and marine sources.
Similar content being viewed by others
Introduction
Development of agricultural activities depends upon the availability of sufficient quantity and quality of water resources. Since the last two decades, groundwater plays an important role in the agricultural sector due to non-availability of surface water in time. In India, irrigation practices utilize about 80 % of the groundwater resources for proper outcome of crop yields, which contributes to increase of national economy.
However, the sources of geogenic (toxic minerals, clay horizons, seawater transgression and incursion, etc.) and non-geogenic (dumping of domestic wastes, leakage of drainage waters and septic tanks, heavy usage of agrochemicals, etc.) origin can deteriorate the quality of groundwater. Back (1966), Drever (1988), Stallard and Edmond (1983), Hem (1991), Karanth (1977) and Subba Rao (2002) explain the causes of changes in the groundwater quality with respect to soil–rock–water interaction, anthropogenic activities and marine sources. Freshwater and salt water interactions are evaluated in Jeju island of South Korea by Kim et al. (2008). Pandey (1983) and Saha (2007) give details on the geochemical characteristics and evolution of groundwater in the Pleistocene deeper and shallow aquifers of South Ganga Plain, Bihar, India. Hydrogeochemical characteristics of groundwater in phreatic aquifers of Alleppey district, Kerala are reported by Shaji et al. (2009). Afroza et al. (2009) evaluated the impact of rock weathering on the groundwater quality with respect to dolomite dissolution and calcium precipitation was discussed in parts of Lower Tista Floodplain, Northwest Bangladesh. Manjusree et al. (2009) explain the groundwater chemistry of the coastal sandy clayey aquifers of Alappuzha district, Kerala, India. Hydrogeochemistry of the coastal aquifers in Tuticorin, Tamil Nadu, India is studied by Mondal et al. (2011) to assess the influence of water–rock interaction, anthropogenic contamination and seawater. Subba Rao et al. (2012) elucidate the chemical characteristics of groundwater from the river basin of Varaha, Visakhapatnam district, Andhra Pradesh, India. Subba Rao et al. (2014a, b) give details on the causes of variation of groundwater chemistry in the coastal region of Chirala–Ongole, Andhra Pradesh, India.
The Western Delta region of the River Godavari, Andhra Pradesh is known as one of the major rice-producing centers in the country (India). But, there are some constraints for developmental activities, including agricultural output. Because, the groundwater occurring in the alluvial deposits in the region is suitable for domestic and irrigation purpose, while that in the floodplain and coastal plain is not fit for any purpose. However, no research has so far been done for assessing the controlling processes of the chemistry of groundwater for taking up suitable remedial measures in the region. Therefore, this region is selected for the present study to evaluate the geochemistry of groundwater for sustainable development of the area.
Study area
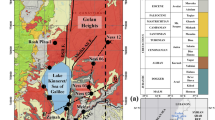
The study region, lying between north latitudes 16°18′05″–17°2′ and east longitudes 81°53′ and 81°52′ (Fig. 1), falls in Survey of India toposheet Nos. 65H/5, 6 and 9–11. It is flanked by upland crystalline terrain on north, Bay of Bengal on south, the river Vasista Godavari on east and the river Upputeru and Lake Kolleru on west. The region experiences a tropical humid type. The minimum temperature is 18 °C in January (winter) and the maximum temperature is 30 °C in May (summer). Mean annual rainfall is 1078 mm. Most rainfall (75 %) received is from southwest monsoon. Mean monthly humidity is 80 % in forenoon and 62 % in afternoon. Mean monthly wind speed ranges from 5.4 km/h in March to 12.7 km/h in July. The annual potential evapotranspiration is 1467 mm.
The study region has a plain land, sloping gently (<3°) toward southeast. Important landforms are valley fills, channels, levee, back swamp, channel, point bar of fluvial and active beach, paleo-beach ridges, back water and tidal flats, spits, mangrove swamps of marine origin (Rengamannar and Pradhan 1961; Nageswara Rao et al. 2005). Kankar (concretion of CaCO3) occurs in the soil zone.
Geologically, the area is underlain by coarse sand with black clay (buried channels zone), black silty clay of recent origin (floodplain zone) and gray/white fine sand of modern beach sediment of paleo-beach ridges and active beach ridge of marine origin (coastal zone; Fig. 2). Lithologs reveal that the topsoil is followed by sticky clay zone, fine sand zone, clayey zone, coarse to medium sand zone and clay–silt zone.
Groundwater occurs under unconfined to semi-confined conditions. Extraction of groundwater is through open dug wells (<10 m depth), filter point wells (10–25 m depth) and tube wells (30–60 m depth). Depth to water level varies from less than 1–22 m. The direction of groundwater flow is NW–SE, following the drainage.
About 95 % of the study area is occupied by agricultural land and the remaining 5 % of the area by tree crops, mangroves, aquaculture practices and small water bodies. The principal crops are paddy, sugarcane and banana. The other crops are cashew nut, mango, coconut, tobacco, turmeric, etc.
Materials and methods
Seventy-two water samples were collected from open dug wells and filter points from the study region during May, 2010 (Fig. 1). Out of which, 29 samples (1–29) belong to buried channel (BC), 30 samples (30–59) to floodplain (FP) and the rest 13 (60–72) to coastal (C) zone.
The pH and electrical conductance (EC) were measured in the field, using their portable meter. The water samples were analyzed for calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), bicarbonate (HCO3 −), chloride (Cl−), sulfate (SO4 2−), nitrate (NO3 −) and fluoride (F−), following the standard water quality methods of APHA (1992). The EC was used to compute the TDS (Hem 1991). The concentrations of TDS and chemical species are expressed in milligrams per liter (mg/L) except pH.
Cation–anion balance was computed, taking their concentrations in milliequivalents per liter (meq/L), which is observed within the limit of ±5 % (Domenico and Schwartz 1990).
Results and discussion
The pH varies from 7.17 to 8.82, being a mean of 7.83 in BC, 7.93 in FP and 7.83 in C zones, indicating alkaline condition (Table 1). Naturally, the pH is controlled by CO2–CO3–HCO3 − equilibrium (Drever 1997). The CO2 with H2O forms H2CO3, resulting in a formation of HCO3 −, which causes a pH (Berner and Berner 1987). It dissolves the salts and/or minerals present in the soils and/or geological formations. Finally, they reach the groundwater body, depending upon the source material. In the present study area, the geological formations of the each zone are intermixed with each other (Fig. 2). Therefore, the pH shows a close variation in the three zones (BC, FP and C). As a result, the hydrogeochemical facies are dominant by HCO3 − ion.
The EC ranges from 266 to 12,900 µS/cm, which is high in C zone (mean 3887 µS/cm), following FP zone (mean 2859 µS/cm) and BC zone (mean 1639 µS/cm). This indicates the variation in the enrichment of salts in the groundwater. The TDS is from 170 to 8256 mg/L. The low TDS is observed from BC (mean 1046 mg/L), medium TDS from FP (mean 1770 mg/L) and high TDS from C (mean 2487 mg/L) zones. On the basis of TDS classification (Fetter 1990), the quality of groundwater shows fresh (TDS <1000 mg/L) to brackish (TDS 1000–10,000 mg/L), the latter being dominant in C zone. The variation in the TDS indicates the influence of canal and river water in BC zone, anthropogenic source in FP and marine source in C zone. The TH is from 120 to 1260 mg/L, being high in C zone (mean 493 mg/L), medium in FP zone (mean 320 mg/L) and low in BC zone (mean 220 mg/L). As per the TH classification (Sawyer and McCarty 1967), the groundwater comes under moderately hard (75–150 mg/L) to very hard (>300 mg/L) categories.
The Ca2+ varies from 8 to 569 mg/L, with a mean of 15, 20 and 65 mg/L in BC, FP and C zones, respectively (Table 1), while the Mg2+ is from 17.39 to 204.20, being a high in C zone (88 mg/L), medium in FP zone (73 mg/L) and low in BC zone (41 mg/L). Relativity, the higher Mg2+ than Ca2+ is a result of ferro-magnesium minerals in soil salts and aquifer sediments, ion exchange between Na+ and Ca2+, precipitation of CaCO3 and seawater intrusion (Todd 1980; Hem 1991; Drever 1997). The Na+ varies from 15.30 to 955.80 mg/L, which is observed to be low from BC (mean 193 mg/L), medium from C (mean 349 mg/L) and high from FP (mean 362 mg/L) zones due to influences of soil salts, deltaic clayey sands, coastal clayey sands, marine salts, anthropogenic activities, etc. on groundwater system. A low K+ (1.42–310.60 mg/L, with a mean of 23, 67 and 92 mg/L in BC, FP and C zones, respectively) indicates its tendency to be fixed by clay minerals (Hem 1991).
The HCO3 − ranges from 84 to 1752 and its mean is low in BC zone (344 mg/L), which is followed by FP (628 mg/L) and FP (752 mg/L) zones. It results from an intense mineral weathering and favoring mineral dissolution (Stumm and Morgan 1996). The Cl− is from 26 to 960 mg/L with its higher concentration (mean 405 mg/L) in C zone, medium concentration (mean 360 mg/L) in BC zone and low concentration (mean 156 mg/L) in FP zone. It derives mainly from the non-lithological sources (domestic waters, septic tanks, irrigation return flows, agrochemicals and marine salts; Todd 1980; Hem 1991; Appelo and Postma 1993; Subba Rao 2002; Nag and Ghosh 2013). The SO4 2− is in between 21.62 and 432.27 mg/L, with a mean of 147 mg/L in BC and FP zones, and 208 mg/L in C zone. The main source of SO4 2− is gypsum, which is used to alter physical and/or chemical properties of soils (Todd 1980). Another source is seawater (Hem 1991). The NO3 − varies from 0.27 to 48 mg/L, which is relatively high (mean 8 mg/L) in C zone compared to BC (mean 4 mg/L) and FP (mean 6 mg/L) zones. The higher concentration of NO3 −, i.e., >10 mg/L, is an indication of man-made pollution (poor sanitary conditions, unlimited application of fertilizers, etc.; (Cushing et al. 1973). The F− is observed to be very low (0.12–0.15 mg/L), which is less than the lowest desirable limit of 0.60 mg/L in the water (BIS 2012).
Further, certain parameters like EC, TDS, Na+, K+, Cl−, HCO3 − and NO3 − show a wide higher range of standard deviations, while others (Ca2+, Mg2+ and SO4 2−) have a lower range of standard deviations in BC, FP and C zones. These suggest the inter-mixing of chemical processes caused by geogenic, anthropogenic and marine origin along with water flow path.
The quality of groundwater shows three types of hydrogeochemical facies based on procedures of Seaber (1962) and Back (1966). They are (i) Na+ > Mg2+ > Ca2+:HCO3 −> Cl−> SO4 2−, (ii) Na+>Mg2+ > Ca2+:Cl−> HCO3 −> SO4 2− and (iii) Mg2+ > Na+ > Ca2+ > or < K+:HCO3 − > Cl− > or < SO4 2−. A dominance of HCO3 − in first and third facies classifies the area as a recharge zone, while that of Cl− in second facies delineates the area as a discharge zone.
Geochemical evolution of groundwater
According to Todd (1980), Hem (1991) and Drever (1997), the chemical species of cations derive mainly from the lithological (geogenic) sources, while those of anions from the non-lithological (anthropogenic) sources. Depending upon the occurrence of source material under climatic conditions, the quality of groundwater varies from place to place. The expected controlling chemical processes in the groundwater are mineral dissolution, leaching of ions, ion exchange, precipitation of ions, evaporation, etc.
Evaporation
In tropical climate, evaporation plays a vital role in the change of groundwater quality. In the present study region, the annual potential evapotranspiration is 1467 mm. The occurrence of kankar in the soil zone supports the evaporation activity in the study region and thereby changes in the groundwater quality. To examine the influence of evaporation on the groundwater, the chemical composition of groundwater of the study region is plotted in Na+ vs Cl− diagram (Fig. 3). Groundwater samples are observed to be close to equiline (1:1) of Na+:Cl− compared to the freshwater evaporation line. It indicates the dissolution of Cl−—salts accumulated by evaporation, as the salts are transferred from groundwater to soil (Drever 1997; Subba Rao 2002). During post-monsoon, the groundwater levels touch the soil zone, which transfers the ions into the soil–water interface.
Saturation index of CaCO3 is also computed for examination of the influence of evaporation on the groundwater. Groundwater in BC zone shows unsaturation (−0.33) with respect to the solid phase of CaCO3 (Table 2), indicating a dissolved state, while it is oversaturation (0.48–0.89) in FP and C zones, resulting a precipitated state, which is further supported by the occurrence of CaCO3 concretions in the soil zone due to tropical humid climate of the study region.
Rock weathering
Rock weathering is a process by which the chemical species can be released into the groundwater through dissolution and leaching. To find out the sources of Ca2+ and Mg2+ in the groundwater, a combination of Ca2+, Mg2+, HCO3 − and SO4 2− is used (Ritchler et al. 1993). The chemical composition of the present study region is plotted in Ca2+ + Mg2+ vs HCO3 − + SO4 2− (Fig. 4). About 96 % of the groundwater samples are observed below the equiline of Ca2+ + Mg2+:HCO3 − + SO4 2−. It indicates an excess of SO4 2− and HCO3 − over Ca2+ and Mg2+ in the groundwater due to input from the weathering of Ca–Mg minerals (calcite, gypsum and plagioclase feldspar present in the sediments (Cerling et al. 1989; Fisher and Mullican 1997; Srinivasamoorthy et al. 2011). The remaining water samples (4 %) fall above the uniline (1:1) of Ca2+ + Mg2+:HCO3 − + SO4 2−, in which the concentrations of Ca2+ and Mg2+ are more than those of SO4 2− and HCO3 −. The extra source of Ca2+ and Mg2+ ions is caused by reverse ion exchange process. From this observation, it is clear that these ions are derived mainly from the weathering of plagioclase feldspar, pyroxenes, amphiboles, biotite and garnet minerals present in the sediments of the study region. Because of the mineral weathering, most groundwater samples (92 %) fall above the fresh water equiline (1:1) of Na+:Cl− (Fig. 3), reflecting the release of Na+ from the mineral weathering (Meyback 1987). If the water derives primarily by mineral weathering, it would have HCO3 − as the most abundant ion, as in the study region (Table 1).
In plot of Na+ + K+ vs total cations (TC), about 92 % of the groundwater samples fall in between the 1:0.5 and 1:1 line of Na+ + K+:TC and the remaining (8 %) water samples are found below the line of 1:0.50 (Fig. 5). This appears to be caused by rock weathering and/or leaching of soil salts (Stallard and Edmond 1983; Subba Rao 2002). Further, it is also observed from the Na+ + K+ vs Cl− + SO4 2− diagram (6), the concentrations of Na+ and K+ increase simultaneously with the increase of Cl− and SO4 2−. This indicates a common source of these ions from the dissolution of soil salts, as also observed in Ganga–Brahmaputra River Basin (Sarin et al. 1989), Delhi Region (Datta and Tyagi 1996), Guntur District (Subba Rao 2002) and Cuddalore District (Srinivasamoorthy et al. 2011) in India (Fig. 6).
The HCO3 − is plotted against TC (Fig. 7) for understanding the influence of dissolution of carbonate and silicate minerals on the groundwater system (Stumm and Morgan 1996; Kim 2003). From the present study region, all groundwater samples fall above the equiline of HCO3 −:TC, but away from its uniline. Broadly, it infers the dissolution of carbonate and silicate minerals in the groundwater. However, the deviation of the groundwater sampling points from the equiline of HCO3 −:TC suggests that the influence of other activities like anthropogenic and marine origin is also involved on the groundwater system. Otherwise, the deviation of water sampling points may not be possible. Thus, there is also a deviation of most of the water sampling points in HCO3 − vs Ca2+ + Mg2+ diagram (Fig. 8), as also reported in Alappuzha District, Kerala, India (Manjusree et al. 2009).
Ion exchange
In Na+ vs Ca2+ diagram (Fig. 9), the groundwater sampling points are away from the uniline of Na+:Ca2+. Especially, they fall mainly toward the concentration of Na+. This explains that the ion exchange of Ca2+ with Na+ (Mercado 1985; Al-Khashman 2007; Saha et al. 2008; Shekhar Gupta et al. 2009) and the influence of soil salts, deltaic clayey sands, coastal clayey sands, anthropogenic activities and marine salts are responsible for the higher concentration of Na+ in the groundwater. Otherwise, we could not expect such a deviation from the equiline of Na+:Ca2+. Further, the higher Mg2+ than Ca2+ is found in the groundwater (Fig. 10). This is a result of dissolution of ferro-magnesium minerals occurring in the soil salts and marine salts (Hem 1991; Subba Rao 2002; Subba Rao et al. 2005).
Hounslow (1995) suggests the possible source of ions from the minerals into groundwater system (Table 2). This further supports the changes in the groundwater chemistry as explained above.
Mechanism controlling groundwater chemistry
In Na+ + K+:Na+ + K+ + Ca2+ and Cl−:Cl− + HCO3 − vs TDS diagrams (Fig. 11), the chemical composition of the groundwater samples of the study region is plotted to assess the influence of precipitation (rainfall), rock and evaporation on groundwater system. The groundwater sampling points spread from the rock domain toward the evaporation one. It indicates that the groundwater chemistry is mainly controlled by rock weathering and subsequently modified by the sources of anthropogenic and marine origin. As the increase of Na+ and Cl− by anthropogenic and marine sources, the groundwater shows a higher TDS. Therefore, the water chemistry moves toward the evaporation domain from the rock domain. These mechanisms obviously explain that the initial groundwater quality is subsequently modified by anthropogenic and marine sources. This further supports the evolution process of the geochemistry of groundwater in the region, as explained in the earlier sections.
Mechanisms controlling the groundwater chemistry (after Gibbs 1970)
Conclusions
The quality of groundwater is mostly brackish and very hard. It shows three facies viz. (i) Na+>Mg2+>Ca2+:HCO3 − > Cl− > SO4 2− > NO3 −, (ii) Na+ > Mg2+ > Ca2+:Cl−> HCO3 − > SO4 2−, and (iii) Mg2+ > Na+ > Ca2+> or < K+:HCO3 − > Cl− > or < SO4 2− facies. The plotting of the chemical composition of the groundwater samples in Ca2+ + Mg2+:HCO3 −, Ca2+ + Mg2+:SO4 2− + HCO3 −, Na+ + K+:TC, Na+ + K+:Cl− + SO4 2−, HCO3 −:TC, HCO3 −:Ca2+ + Mg2+, Na+:Cl− and Na+:Ca2+ diagrams suggests that the quality of groundwater is controlled by rock weathering, mineral dissolution, evaporation and ion exchange. The possible source of ions altered the original composition of groundwater mostly by dolomite dissolution and calcium precipitation and weathering of plagioclase and other ferro-magnesium minerals present in the sediments. The sources of anthropogenic and marine origin are the supplementary factors for brackish water quality. This is further supported by Gibbs mechanisms that control the aquifer chemistry. Thus, the present study suggests that the original chemical composition has been affected by geogenic and subsequently modified by the impact of anthropogenic and marine sources.
References
Afroza R, Mazumder HQ, Chowdhury S, Jahan S, Kazi MAI (2009) Hydrochemistry and origin of salinity in groundwater in parts of lower Tista Floodplain, Northwest Bangladesh. J Geol Soc India 74:223–232
Al-Khashman O (2007) Study of water quality of springs in Petra region, Jordan, a three-year follow-up. Water Resources Management 21(7):1145–1163
APHA (1992) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC, p 326
Appelo CAJ, Postma D (1993) Geochemistry, groundwater, and pollution. A Balkema Publication, Rotterdam
Back W (1966) Hydrochemical facies and groundwater flow pattern in Northern Part of Atlantic Coastal Plain. US Geological Survey Paper, 498A, p 42
Berner EK, Berner RA (1987) The global water cycle, geochemistry and environment. Prentice-Hall, New Jersey
BIS (2012) Indian standard specifications for drinking water. IS:10500, Bureau of Indian Standards, New Delhi
Cerling TE, Pederson BL, Dam KLV (1989) Sodium and calcium ion exchanging in weathering of shale, implication of global weathering. Budget 17:552–554
Cushing EM, Kantrowitz IH, Taylor KR (1973) Water resources of the Delmarva Peninsular. US Geological Survey Professional Paper 822, Washington DC
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater flow regime. J Geol Soc India 47:179–188
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology. Wiley, New York, p 824
Drever JI (1988) The geochemistry of natural water. Pearson Education Canada, p 437
Drever JI (1997) The geochemistry of natural waters (p 388). Prentice Hall Inc, New Jersey, p 379
Fetter CW (1990) Applied hydrogeology. CBS Publishers & Distributors, New Delhi, p 592
Fisher RS, Mullican WF (1997) Hydrochemical evolution of sodium-sulphate and sodium-chloride groundwater beneath the northern Chihuahuan desert; Trans-Pecos, Texas, USA. Hydrogeol J 5:4–16
Gibbs RJ (1970) Mechanism controlling world’s water chemistry. Science 170:1088–1090
Gupta S, Dandele PS, Verma MB, Maithani PB (2009) Geochemical assessment of groundwater around Macherla–Karempudi area, Guntur district, Andhra Pradesh. J Geol Soc India 73:202–212
Hem JD (1991) Study and interpretation of the chemical characteristics of natural water. U.S. Geological Survey Water Supply Paper 2254. Scientific Publishers, Jodhpur, p 264
Hounslow AW (1995) Water quality data analysis and interpretation. Lewis Publishers, New York, pp 45–128
Karanth KR (1977) Groundwater assessment, development and management. Mc Graw-Hill, New Delhi, p 720
Kim K (2003) Long-term disturbance of groundwater chemistry following well installation. Ground Water 41:780–789
Kim HJ, Wang B, Ding Q (2008) Changes in arid climate over north China detected by Koppen climate classification. J Meteor Soc Japan 86(6):981–990
Manjusree TM, Joseph Sabu, Thomas Jobin (2009) Hydrogeochemistry and groundwater quality in the coastal sandy clay aquifers of Alappuzha district, Kerala. J Geol Soc India 74:459–468
Mercado A (1985) The use of hydrochemical pattern in carbonate sand and sandstone aquifers to identify intrusion and flushing of saline water. Groundwater 23:335–344
Meyback M (1987) Global chemical weathering surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Mondal NC, Singh VP, Singh S, Saxena VS (2011) Hydrochemical characteristics of coastal aquifer from Tuticorin, Tamil Nadu, India. Environ Monit Assess 175:531–550
Nag SK, Ghosh P (2013) Variation in groundwater levels and water quality in Chhatna block, Bankura district, West Bengal—a GIS approach. J Geol Soc India 81:261–280
Nageswara Rao K, Sadakata N, Hemamalini B, Takayas K (2005) Sedimentation processes and asymmetric development of the Godavari delta, India; River Deltas—concepts, models, and examples. SEPM Spec Publ 83:435–451
Pandey KS (1983) Hydrogeology and groundwater potential in the marginal alluvial tract of Bhagalpur and Mongger districts, Bihar. In: Proceedings of Sem. Assess. Dev. Man. Groundwater Res., Central Groundwater Board, New Delhi, India, vol 2, pp 121–132
Rengamannar V, Pradhan PK (1961) Geomorphology and evolution of Godavari delta. In: R. Vaidyanadhan (ed), Quaternary Deltas of India, vol 22. Member of Geological Society, India, pp 51–56
Ritchler BC, Kreittler CW, Bledsoe BE (1993) Geochemical techniques for identifying sources of groundwater salinization. CRC, New York, p 272
Saha D (2007) The aquifer system and evaluation of its hydraulic parameters in parts of South Ganga Plain. J Geol Soc India 69:1031–1041
Saha D, Dhar YR, Sikdar PK (2008) Geochemical evolution of groundwater in the Pleistocene aquifers of South Ganga Plain, Biha. J Geol Soc India 71:473–482
Sarin MM, Krishnaswamy S, Dilli K, Somayajulu BLK, Moore WS (1989) Major-ion chemistry of the Ganga-Brahmaputra river system: weathering processes and fluxes to the Bay of Bengal. Geochim et Cosmochim Acta 53:997–1009
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers. McGraw- Hill, New York
Seaber PR (1962) Cation hydrochemical facies of groundwater in the English town formation, New Jersey. U.S. Geological Survey Professional Paper 450 B, pp 124–126
Shaji E, Vinayachandran N, Thambi DS (2009) Hydrogeochemical characteristics of groundwater in coastal phreatic aquifers of Alleppy district, Kerala. J Geol Soc India 74:585–590
Srinivasamoorthy M, Vasanthavigar S, Chidambaram S, Anandan P, Sharma VS (2011) Characterization of groundwater chemistry in an eastern coastal area of Cuddalore district, Tamil Nadu. J Geol Soc India 78:549–558
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon River—the influence of the geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley-Interscience, New York, p 780
Subba Rao N (2002) Geochemistry of groundwater in parts of Guntur District, Andhra Pradesh, India. Environ Geol 41:552–562
Subba Rao N, Surya Rao P, Venktram Reddy G, Nagamani M, Vidyasagar G, Satyanarayana NLVV (2012) Chemical characteristics of groundwater and assessment of groundwater quality in Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ Monit Assess 184:5189–5214
Subba Rao N, Vidyasagar G, Surya Rao P, Bhanumurthy P (2014a) Chemistry and quality of groundwater in a coastal region of Andhra Pradesh. Appl Water Sci, India. doi:10.1007/s13201-014-0244-0
Subba Rao N, Vidyasagar G, Surya Rao P, Bhanumurthy P (2014b) Assessment of hydrogeochemical processes in a coastal region: application of multivariate statistical model. J Geol Soc India 84:494–500
Subba Rao N, Saroja Nirmala I, Suryanarayana K (2005) Groundwater quality in a coastal area—a case study from Andhra Pradesh, India. Environ Geol 48:543–550
Todd DK (1980) Groundwater hydrology. Wiley Publications, New York
Acknowledgments
The authors thank anonymous reviewers and editor of the journal for their valuable suggestions in bringing the manuscript in the present form.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nageswara Rao, P.V., Appa Rao, S. & Subba Rao, N. Geochemical evolution of groundwater in the Western Delta region of River Godavari, Andhra Pradesh, India. Appl Water Sci 7, 813–822 (2017). https://doi.org/10.1007/s13201-015-0294-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0294-y