Abstract
In the present work, CuO nanostructures with different morphologies were synthesized by a simple one-pot reflux condensation approach using different alkaline precursors. Structural analysis by X-ray diffraction and Fourier transform infrared spectroscopy revealed the formation of single phase CuO with a monoclinic crystal structure. Morphological analysis by scanning electron microscopy showed the formation of spindle-shaped and flower-like CuO architecture when NaOH and NH4OH were used as alkaline precursors, respectively. The flower-like CuO architecture is found to be made up of 2D nanosheets as building blocks, which were self-assembled to form spherical assemblies. Optical analysis by UV–VIS diffused reflectance spectroscopy showed blue-shift in the optical band gap due to quantum confinement effect. Photoluminescence spectra showed both UV and visible emission. The plausible growth mechanism for the formation of different CuO nanostructures was proposed.
Similar content being viewed by others
Introduction
In recent years, controlled synthesis of inorganic materials with well-defined morphologies has attracted considerable interest since the morphological diversity of inorganic materials has a significant impact on their functional diversification and potential applications. The ability to tune the size and shape of inorganic materials is of extraordinary importance because their electronic structure, bonding, surface energy, and chemical reactivities are directly related to their surface morphology (Xia et al. 2010). Copper oxide (CuO) is a narrow band gap p-type multifunctional semiconductor, and has been recognized as a technologically important material for a variety of practical applications, such as catalysis, batteries, magnetic storage media, solar energy conversion, gas sensing and field emission transistors (Frietsch et al. 2000; Maruyama 1998; Chowdhuri et al. 2004; Chen et al. 2003; Zhang et al. 2006). In addition, CuO-based materials are well-known with regard to their high-temperature superconductivity and giant magnetoresistance (Wen et al. 2005; Liu et al. 2006). The prospect of potential applications of CuO nanostructures has led to substantial research and development efforts to form various types of nanostructures. So far, a variety of CuO nanostructures including nanoparticles, nanoneedles, nanowhiskers, nanowires, nanobelts, and nanosheets have been obtained successfully by various methods namely alcothermal (El-Trass et al. 2012), hydrothermal (Dar et al. 2008), electrodeposition (Mukherjee et al. 2011), thermal oxidation (Kaur et al. 2006), reverse micelle (Yuasa et al. 2009), and microwave synthesis (Zhu and Qian 2010). To some extent, all the above methods possess some disadvantages, such as high processing temperature, complex experimental procedures, multi-step reactions, sophisticated equipments, time consumption, and thus limiting the large-scale applications. Therefore, developing a simple, inexpensive, and robust approach for the rational synthesis of CuO nanostructures under mild reaction conditions is still a challenging task, and is highly desired for exploring CuO in device applications. In this context, in the present work, we explore a facile and environmentally benign reflux condensation approach to synthesis CuO nanostructures with different morphologies without using any surfactants or organic solvents. This approach has advantages such as simplicity, safety, environmentally benign synthetic conditions, good potential for scale up, and also provides an alternative pathway for economical synthesis of CuO nano/microstructures. The morphology of CuO particles was tuned by using ammonia (25 %, NH4OH) and sodium hydroxide (NaOH) as alkaline precursors. The structural and morphological properties of CuO particles were investigated using XRD, FTIR, and SEM, and the optical properties were investigated by UV–VIS DRS and PL measurements, and the plausible growth mechanism for the formation of different CuO nanostructures was proposed.
Experimental details
CuO synthesis
All the reagents used in the experiments were analytically pure, and were used without further purification. In a distinctive synthesis, 0.1 M of copper nitrate trihydrate (Cu(NO)3·3H2O) was prepared in 100 ml of deionized water and stirred constantly until a homogeneous blue color solution was obtained. Then, 5 M aqueous ammonia solution was injected vigorously, and the resulting mixture was refluxed at 100 °C for 12 h. After the reaction is complete, the product was cooled to room temperature naturally, and a great amount of black precipitate was obtained. The precipitate was centrifuged and washed with methanol several times to remove the by-products and impurities. Finally, the black product was dried in air at 100 °C for 2 h. Similarly, CuO particles were synthesized using 5 M aqueous NaOH solution instead of NH4OH solution and the procedure was repeated as mentioned above. Herein, the products obtained using NH4OH and NaOH as alkaline precursors were designated as sample ‘A’ and ‘B’, respectively.
Characterization
XRD pattern was obtained using Rigaku Ultima III X-ray diffractometer equipped with CuKα radiation (λ = 1.5406 Å). FTIR spectra were recorded using PerkinElmer FTIR spectrometer in the spectral range 400–2,000 cm−1. Morphological imaging was carried out using JEOL JSM-6390LV SEM. The optical band gap was estimated from UV–VIS DRS using JASCO V-670 double beam spectrophotometer in the wavelength range 350–1,400 nm. PL spectra were recorded using a JOBIN–YVON FLUROLOG-3-11 spectrofluorometer.
Results and discussion
Structural analysis
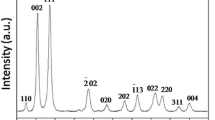
The crystal structure and phase purity of the products were analyzed by XRD. Figure 1 shows the XRD pattern of the products synthesized using different alkaline precursors. The diffraction peak positions are identical for both the samples and all the diffraction peaks could be indexed as monoclinic crystal structure of CuO, which are in good agreement with the standard JCPDS data (card no. 45-0937). No other impurity peak is detected in the XRD pattern indicating the high purity of the as-synthesized samples. The diffraction peaks of sample ‘A’ shows larger intensity with smaller full width at half maxima (FWHM) indicating highly crystalline nature compared to sample ‘B’. Both the samples exhibit considerably broad diffraction peaks indicating the formation of nanosized particles (Bhalerao-Panajkar et al. 2011). The lattice parameters of the products synthesized using different alkaline precursors are calculated from the XRD pattern, and are given in Table 1. The calculated lattice parameter values are in good agreement with the standard values of CuO. The mean crystallite size (D) was evaluated using Debye–Scherrer’s formula (Al-Gaashani et al. 2011) given by Eq. (1),
where k is a shape factor (0.90), λ is the wavelength of CuKα radiation, β is the FWHM, and θ is angle of reflection. The mean crystallite sizes of samples A and B were found to be 19 and 26 nm, respectively.
The composition and quality of the products were analyzed by FTIR. Figure 2 shows the typical FTIR spectra of sample A. The FTIR spectrum showed strong absorptions between 400 and 610 cm−1, and weak absorptions between 1,300 and 1,700 cm−1. The spectra show absorption peaks around 407, 433, 479, 529, and 603 cm−1 corresponding to the characteristic stretching vibrations of Cu–O bond in the monoclinic CuO (Erdogan and Gullu 2010). The characteristic peaks around 432 cm−1 was ascribed to the Cu–O stretching along the [2 0 2] directions, and the peaks around 530 and 602 cm−1 was attributed to the Cu–O stretching along [−2 0 2] direction (Dar et al. 2008; Anu Prathap et al. 2012). The weak absorption bands between 1,300 and 1,700 cm−1 are mainly ascribed to the chemisorbed and/or physisorbed H2O and CO2 molecules on the surface of nanostructured CuO. The peak at ~1,638 cm−1 was due to the bending vibration of water (Karthik et al. 2011). Similarly, the FTIR spectra of sample B (not shown here) exhibit peaks at 409, 493, 533, and 1,649 cm−1. Also the absence of absorption peak at 615 cm−1 corresponding to the infrared active mode of Cu2O (Erdogan and Gullu 2010; Karthik et al. 2011), further confirms that the as-synthesized products comprise purely CuO phase without any trace of Cu2O being present.
Morphological analysis
Figure 3 shows the SEM images of CuO products synthesized using different alkaline precursors. It can be observed that the alkaline precursors have substantial impact on the growth of CuO nanostructures. SEM images of sample ‘A’ (Fig. 3a) synthesized using NH4OH as alkaline precursor showed the formation of 3D flower-like CuO microspheres with an average diameter of about 1–2 μm. Higher magnification in the inset shows that the flower-like hierarchical microspheres are composed of 2D nanosheet subunits as building blocks, which were self-organized to form spherical assemblies. SEM images of sample ‘B’ (Fig. 3b) synthesized using NaOH as alkaline precursor showed spindle-like particles composed of self-organized nanorods.
Growth mechanism
On the basis of above experimental result, we propose the following plausible mechanism for the formation of flower- and spindle-like CuO architecture. In the present work, CuO particles were synthesized by the thermal decomposition of [Cu(NH3)4]2+ or Cu(OH)2 precursor directly under hydrothermal condition. During the formation of CuO particles, the following reactions might occur:
When ammonia was added to the solution, formation of copper oxide takes place through the ammonium complex [Cu(NH3)4]2+. Initially at room temperature, copper ions react with ammonia to form a deep blue solution containing [Cu(NH3)4]2+ complex ions (Eq. 2). With the increase in temperature, the complex hydrolyzed and reacts with OH− ions in the solution rapidly in short time to form CuO nanoparticles (Eq. 3). Since the nanoparticles are highly reactive and possess high surface energy, they tend to self-aggregate or self-organize slowly to form stable CuO nanosheets. With the prolonged reaction time, the nanosheets undergo oriented attachment to form 3D flower-like hierarchical microspheres. On the other hand, when NaOH was used as precursor, Cu2+ generated in the solution combines with OH− to form Cu(OH)2 nanoparticles which acts as nuclei (Eq. 4). Driven by the minimization of interfacial energy, the Cu(OH)2 nanoparticles aggregate and assemble themselves to form nanorods. As orthorhombic Cu(OH)2 is a layered structure, the growth rate is anisotropic, leading to the formation of one-dimensional structure. During heating process, the Cu(OH)2 nanorods loose H2O molecules and transform into CuO nanorods (Eq. 5). With the prolonged reaction time, the CuO nanorods aggregate into spindle-shaped particles because of their high surface energy or van der Waals forces. Hence, it can be implied that the alkaline precursor added to the system has a significant influence on the final morphology of the CuO nanostructures.
Optical properties
The optical band gap of CuO was estimated from UV–VIS DRS. Figure 4 shows the UV–VIS DRS of CuO particles synthesized using different alkaline precursors. The steep shape of the absorption between 750 and 950 nm indicates that the light absorption is due to the intrinsic band-gap transition. The wavelength of absorption edge (λg) was determined by extrapolating the horizontal and sharply rising portions of the curve to the abscissa at zero absorption (Wang et al. 2011). The absorption edge of samples ‘A’ and ‘B’ was found at 929 and 960 nm, respectively. The energy band gap of CuO was found using Eq. (6),
The band gap of CuO synthesized using NH4OH and NaOH as alkaline precursors was found to be 1.35 and 1.29 eV, respectively, and the values are in good agreement with earlier report (Yang and He 2011). The observed band-gap values are larger than the bulk CuO (1.2 eV), which can be attributed to the well-known quantum confinement effect resulting from nanosized crystals (Anu Prathap et al. 2012; Jana et al. 2010). Even though the synthesized CuO superstructures have diameters in the micrometer range, we observed that the subunits are formed by the accumulation of several thousand of CuO nanoparticles which could exert quantum confinement.
Figure 5 shows the PL spectra of CuO synthesized using different alkaline precursors. Both the samples exhibit similar peak position but with different intensities. PL spectra of the samples exhibits UV emission peaks at 324, 340, 354 nm, and visible emission peaks in the violet (402 nm) and blue regions (426 and 451 nm). The origin of luminescence in CuO still remains contradictory, and the near band edge emission of CuO was observed at 365 nm (El-Trass et al. 2012), 300 nm (Aslani 2011), 395 nm (Mukherjee et al. 2011), and 467 nm (Erdogan and Gullu 2010). Nevertheless, the enhanced PL intensity indicates the good crystalline nature of sample A.
Conclusion
CuO nanostructures with different morphologies were synthesized by cost-effective reflux condensation approach without using any surfactant or templates. CuO spindle- and flower-shaped hierarchical architectures were obtained by simply varying the alkaline precursors. XRD, SEM, UV–VIS DRS and PL spectra were used to characterize the structure, morphology, and phase of the synthesized product. Owing to its great chemical flexibility and synthetic tenability, the present route provides a simple and green pathway of synthesizing 2D and 3D CuO nanostructures. Also, it can be anticipated that the current synthesis strategy can be extended for the preparation of other technologically important semiconductors.
References
Al-Gaashani R, Radiman S, Tabet N, Razak Daud A (2011) Synthesis and optical properties of CuO nanostructures obtained via a novel thermal decomposition method. J Alloys Compd 509:8761–8769. doi:10.1016/j.jallcom.2011.06.056
Anu Prathap MU, Kaur B, Srivastava R (2012) Hydrothermal synthesis of CuO micro-/nanostructures and their applications in the oxidative degradation of methylene blue and non-enzymatic sensing of glucose/H2O2. J Colloid Interface Sci 370:144–154. doi:10.1016/j.jcis.2011.12.074
Aslani A (2011) Controlling the morphology and size of CuO nanostructures with synthesis by solvo/hydrothermal method without any additives. Phys B 406:150–154. doi:10.1016/j.physb.2010.10.017
Bhalerao-Panajkar RS, Shirolkar MM, Das R, Maity T, Poddar P, Kulkarni SK (2011) Investigations of magnetic and dielectric properties of cupric oxide nanoparticles. Solid State Commun 151:55–60. doi:10.1016/j.ssc.2010.10.024
Chen J, Deng S, Xu N, Zhang W, Wen X, Yang S (2003) Temperature dependence of field emission from cupric oxide nanobelt films. Appl Phys Lett 83:746–748. doi:10.1063/1.1595156
Chowdhuri A, Gupta V, Sreenivas K, Kumar R, Mozumdar S, Patanjali PK (2004) Response speed of SnO2-based H2S gas sensors with CuO nanoparticles. Appl Phys Lett 84:1180–1182. doi:10.1063/1.1646760
Dar MA, Kim YS, Kim WB, Sohn JM, Shin HS (2008) Structural and magnetic properties of CuO nanoneedles synthesized by hydrothermal method. Appl Surf Sci 254:7477–7481. doi:10.1016/j.apsusc.2008.06.004
El-Trass A, ElShamy H, El-Mehaseeb I, El-Kemary M (2012) CuO nanoparticles: Synthesis, characterization, optical properties and interaction with amino acids. Appl Surf Sci 258:2997–3001. doi:10.1016/j.apsusc.2011.11.025
Erdogan IY, Gullu O (2010) Optical and structural properties of CuO nanofilm: its diode application. J Alloys Compd 492:378–383. doi:10.1016/j.jallcom.2009.11.109
Frietsch M, Zudock F, Goschnick J, Bruns M (2000) CuO catalytic membrane as selectivity trimmer for metal oxide gas sensors. Sens Actuators B Chem 65:379–381. doi:10.1016/S0925-4005(99)00353-6
Jana S, Das S, Das NS, Chattopadhyay KK (2010) CuO nanostructures on copper foil by a simple wet chemical route at room temperature. Mater Res Bull 45:693–698. doi:10.1016/j.materresbull.2010.02.014
Karthik K, Victor Jaya N, Kanagaraj M, Arumugam S (2011) Temperature-dependent magnetic anomalies of CuO nanoparticles. Solid State Commun 151:564–568. doi:10.1016/j.ssc.2011.01.008
Kaur M, Muthe KP, Despande SK, Choudhury S, Singh JB, Verma N, Gupta SK, Yakhmi JV (2006) Growth and branching of CuO nanowires by thermal oxidation of copper. J Cryst Growth 289:670–675. doi:10.1016/j.jcrysgro.2005.11.111
Liu Y, Chu Y, Li M, Dong L (2006) In situ synthesis and assembly of copper oxide nanocrystals on copper foil via a mild hydrothermal process. J Mater Chem 16:192–198. doi:10.1039/B512481F
Maruyama T (1998) Copper oxide thin films prepared by chemical vapor deposition from copper dipivaloylmethanate. Sol Energy Mater Sol Cells 56:85–92. doi:10.1016/S0927-0248(98)00128-7
Mukherjee N, Show B, Maji SK, Madhu U, Bhar SK, Mitra BC, Khan GG, Mondal A (2011) CuO nano-whiskers: electrodeposition, Raman analysis, photoluminescence study and photocatalytic activity. Mater Lett 65:3248–3250. doi:10.1016/j.matlet.2011.07.016
Wang J, Fan XM, Wu DZ, Dai J, Liu H, Liu HR, Zhou ZW (2011) Fabrication of CuO/T-ZnOw nanocomposites using photo-deposition and their photocatalytic property. Appl Surf Sci 258:1797–1805. doi:10.1016/j.apsusc.2011.10.048
Wen XG, Xie YT, Choi CL, Wan KC, Li XY, Yang SH (2005) Copper-based nanowire materials: templated syntheses, characterizations, and applications. Langmuir 21:4729–4737. doi:10.1021/la050038v
Xia J, Li H, Luo Z, Wang K, Yin S, Yan Y (2010) Ionic liquid-assisted hydrothermal synthesis of three-dimensional hierarchical CuO peachstone-like architectures. Appl Surf Sci 256:1871–1877. doi:10.1016/j.apsusc.2009.10.022
Yang M, He J (2011) Fine tuning of the morphology of copper oxide nanostructures and their application in ambient degradation of methylene blue. J Colloid Interface Sci 355:15–22. doi:10.1016/j.jcis.2010.11.022
Yuasa M, Masaki T, Kida T, Shimanoe K, Yamazoe N (2009) Nano-sized PdO loaded SnO2 nanoparticles by reverse micelle method for highly sensitive CO gas sensor. Sens Actuators B Chem 136:99–104. doi:10.1016/j.snb.2008.11.022
Zhang JT, Liu JF, Peng Q, Wang X, Li YD (2006) Nearly monodisperse Cu2O and CuO nanospheres: preparation and applications for sensitive gas sensors. Chem Mater 18:867–871. doi:10.1021/cm052256f
Zhu J, Qian X (2010) From 2-D CuO nanosheets to 3-D hollow nanospheres: interface-assisted synthesis, surface photovoltage properties and photocatalytic activity. J Solid State Chem 183:1632–1639. doi:10.1016/j.jssc.2010.05.015
Acknowledgments
The authors are thankful to the Secretary and the Management of Kongunadu Arts and Science College, Coimbatore, India for their support and also for providing necessary facilities to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mageshwari, K., Sathyamoorthy, R. Organic free synthesis of flower-like hierarchical CuO microspheres by reflux condensation approach. Appl Nanosci 3, 161–166 (2013). https://doi.org/10.1007/s13204-012-0116-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0116-6