Abstract
Nanocrystalline titanium dioxide (TiO2) thin film was successfully prepared by simple electrodeposition method from alkaline aqueous solution containing potassium titanium oxalate and hydroxylamine. Surface characterization of the electrodeposited films indicates the formation of crystalline TiO2. The dye solar cell constructed from dye-modified electrodeposited TiO2 film achieved an overall light-to-electricity conversion efficiency of 2.1 % under 1 sun illumination, indicating its high potential as a photoelectrode material for the DSCs.
Similar content being viewed by others
Introduction
Nanostructured semiconductor oxide materials have recently attracted a great deal of attention owing to their excellent optical, chemical, photoelectrochemical and electronic properties. Among the metal oxides, nanocrystalline titanium dioxide (TiO2) is one of the most investigated materials due to its important applications in environmental cleanup (Andersson et al. 2002; Karuppuchamy and Jeong 2005; Karuppuchamy et al. 2006), photocatalysis (Kawahara et al. 2007a, b; Miyazaki et al. 2008) and dye-sensitized solar cells (DSC) (O’Regan and Graetzel 1991; Karuppuchamy et al. 2001, 2002, 2006; Oekermann et al. 2004; Okada et al. 2005; Wessels et al. 2008; Park et al. 2011; Wang and Kerr 2011). For these industrial applications, the preparation of optically transparent TiO2 films in a large area and the improvement of their photo-functional properties would be crucial tasks. So far thin films of transparent TiO2 are classically deposited from the vapor phase. However, these methods have high costs, and the preparation of films in a large area is technically difficult. Electrodeposition is a promising technique for the preparation of transparent nanocrystalline semiconductor oxide thin films in large area substrates. Therefore, our recent research has been focused on the development of nanostructured TiO2 films by simple and low-cost electrochemical method (Karuppuchamy et al. 2001, 2002; Oekermann et al. 2004). In this study, we describe a widely applicable and relatively simple electrochemical approach for the successful preparation of nanocrystalline TiO2 thin film. Further, the promising applications of electrodeposited TiO2 thin films in dye-sensitized solar cells also reported.
Experimental
A conventional three-electrode single compartment cell was used with an ITO-coated glass as a working electrode, a Pt foil as a counter electrode and an Ag/AgCl as a reference electrode. The deposition bath consisted of an aqueous solution of 0.1 M K2[TiO(C2O4)2] and 1 M Hydroxylamine. The pH of this solution was adjusted to 8.0 by the addition of KOH. Cathodic electrodeposition was carried out in the potential range between −1.0 and −1.2 V (vs. Ag/AgCl) which led to the formation of TiO2 film on the ITO electrode. Subsequently, the electrodeposited film was subjected to heat-treatment at 450 °C for 1 h in air to obtain crystalline anatase TiO2 thin film. The resultant films were characterized by X-ray diffractometry (XRD) and scanning electron microscopy (SEM).
For dye-sensitized photoelectrochemical investigations, a sandwich-type configuration was employed. A Pt-coated glass slide was used as a counter electrode, and 0.1 M LiI + 0.05 M I2 + 0.6 M dimethylpropylimidazolium iodide + 0.5 M tert-butylpyridine in methoxypropionitrile was used as electrolyte. The dye tetrabutylammonium cis-di(thiocyanato)-N,N′-bis-(4-carboxylato-4′-carboxylic acid-2,2′-bipyridine)ruthenate(II) (N719, Solaronix) was adsorbed by immersing the electrodeposited TiO2 films in a 0.3 mM ethanolic solution of the dye. The dye-coated electrodes were rinsed quickly with acetonitrile and used as such for photovoltaic measurements. Photocurrent–voltage characteristics of solar cells were measured using IV curve analyzer and solar simulator equipped with AM 1.5 filter was used as a light source.
Results and discussion
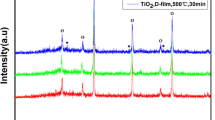
Figure 1 shows a typical XRD pattern of cathodically electrodeposited and heat-treated TiO2 sample. The XRD pattern of the as-deposited film indicates the formation of reduced form of TiO2, i.e. Ti5O9 (Fig. 1a). The reduced form of TiO2 was completely converted into anatase TiO2 after the heat treatment. Optical properties of the electrodeposited films were studied by UV–vis absorption spectra (Fig. 2). The electrodeposited pure TiO2 thin film exhibits a gradual increase of absorbance along with decrease in wavelength due to light scattering and a sharp increase of band gap absorption below approximately 380 nm. The TiO2/N719 thin film shows absorption in visible range peaking at 520 nm, somewhat shifted towards a longer wavelength compared to that of the solution. This may be due to the formation of ester-like linkage for the anchoring of the dyes with carboxylic acid groups on TiO2 surface.
Figure 3 shows the photocurrent–voltage characteristic of the cell constructed from electrodeposited TiO2 porous film. The solar cell generates a short-circuit photocurrent density (Jsc) of 6.5 mA/cm2, an open-circuit voltage (Voc) of 0.74 V, fill factor (F.F.) of 0.43 and an overall 2.1 % light-to-electricity conversion efficiency (η) under 1 sun illumination. The insufficient efficiency of the present material could be partly due to the low amount of dye in the film. The loaded dye in the present case was indeed much lower (9.7 × 10−9 mol/cm2) than the optimum value (exceeding 1 × 10−7 mol/cm2), which is attributable to the low surface area of our films. Further investigations to improve the photoelectrochemical performance of the electrodeposited TiO2 are under way.
Conclusions
Nanocrystalline TiO2 thin film was successfully prepared by simple electrodeposition method from alkaline aqueous solution containing potassium titanium oxalate and hydroxylamine. The dye solar cell constructed from dye-modified electrodeposited TiO2 film achieved an overall light-to-electricity conversion efficiency of 2.1 % under 1 sun illumination, indicating its high potential as a photoelectrode material for the DSCs.
References
Andersson M, Osterlund L, Ljungstrom S, Palmqvist A (2002) Preparation of nanosize anatase and rutile TiO2 by hydrothermal treatment of microemulsions and their activity for photocatalytic wet oxidation of phenol. J Phys Chem B 106:10674–10679
Karuppuchamy S, Jeong J (2005) Super-hydrophilic amorphous titanium dioxide thin film deposited by cathodic electrodeposition. Mater Chem Phys 93:251–254
Karuppuchamy S, Amalnerkar DP, Yamaguchi K, Yoshida T, Sugiura T, Minoura H (2001) Cathodic electrodeposition of TiO2 thin films for dye-sensitized photoelectrochemical applications. Chem Lett 30(1):78–79
Karuppuchamy S, Nonomura K, Yoshida T, Sugiura T, Minoura H (2002) Cathodic electrodeposition of oxide semiconductor thin films and their application to dye-sensitized solar cells. Solid State Ionics 151:19–27
Karuppuchamy S, Jeong J, Amalnerkar DP, Minoura H (2006a) Photoinduced-hydrophilicity of titanium dioxide thin films deposited by cathodic electrodeposition. Vaccum 80:494–498
Karuppuchamy S, Iwasaki M, Minoura H (2006b) Electrochemical properties of electrosynthesized TiO2 thin films. Appl Surf Sci 253:2924–2929
Kawahara T, Kuroda T, Matsui H, Mishima M, Karuppuchamy S, Seguchi Y, Yoshihara M (2007a) Electronic properties of calcined materials from a scandium-O-phenylene-O-yttrium-O- phenylene hybrid copolymer. J Mater Sci 42:3708–3713
Kawahara T, Miyazaki H, Karuppuchamy S, Matsui H, Ito M, Yoshihara M (2007b) Electronic nature of vanadium nitride–carbon cluster composite materials obtained by the calcination of oxovanadylphthalocyanine. Vaccum 81:680–685
Miyazaki H, Matsui H, Nagano T, Karuppuchamy S, Ito S, Yoshihara M (2008) Synthesis and electronic behaviors of TiO2/carbon clusters/Cr2O3 composite materials. Appl Surf Sci 254:7365–7369
O’Regan B, Graetzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Oekermann T, Karuppuchamy S, Yoshida T, Schelettwein D, Woehrle D, Minoura H (2004) Electrochemical self-assembly of ZnO/SO3EtPTCDI hybrid photoelectrodes. J Electrochem Soc 151(1):C62–C68
Okada N, Karuppuchamy S, Kurihara M (2005) An efficient dye-sensitized photoelectrochemical solar cell made from CaCO3-coated TiO2 nanoporous film. Chem Lett 34(1):16–17
Park H, Kim WR, Jeong H-T, Lee J–J, Kim H-G, Choi W-Y (2011) Fabrication of dye-sensitized solarcells by transplanting highly ordered TiO2 nanotube arrays. Sol Energy Mater Sol Cells 95:184–189
Wang B, Kerr LL (2011) Dye sensitized solar cells on paper substrates. Sol Energy Mater Sol Cells 95:2531–2535
Wessels K, Maekawa M, Rathousky J, Yoshida T, Wark M, Oekermann T (2008) Highly porous TiO2 films from anodically deposited titanate hybrids and their photoelectrochemical and photocatalytic activity. Microporous Mesoporous Mater. 111:55–61
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Karuppuchamy, S., Andou, Y. & Endo, T. Preparation of nanostructured TiO2 photoelectrode for flexible dye-sensitized solar cell applications. Appl Nanosci 3, 291–293 (2013). https://doi.org/10.1007/s13204-012-0140-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0140-6