Abstract
Synthesis of nanoparticles of cadmium selenide (CdSe) was carried out using solvothermal method with cadmium nitrate and sodium selenite as precursors. Hydrazine hydrate and ethylenediamine tetra acetic acid were used as the capping agent to control the size of the nanoparticles. As their size decreases to their Bohr radius (usually around a few nanometers), all electronic properties change, and equally important, become dependent on size. In this size, a semiconductor nanoparticle transition occurs in which the electrons and holes are confined beyond their natural Bohr radius. The properties become dependent not only on size, but also on shape. The crystalline nature and particle size of the samples were characterized by Powder X-ray diffraction analysis (XRD). The morphology of prepared CdSe nanoparticles was studied by scanning electron microscope. Dielectric studies were carried out for the pelletized sample of CdSe nanoparticles. The ac conductivity of CdSe nanoparticle has been studied. The obtained results are discussed.
Similar content being viewed by others
Introduction
The semiconductor nanoparticles belong to the state of matter in transition between molecules and bulk solids in which the relevant physical dimensions changes on the length of a few to a few hundred nanometers. Both equilibrium and dynamic properties of nanomaterials can be very different from those of their corresponding bulk materials or isolated atoms and molecules. The dielectric constant of a semiconductor is one among its most important properties. Its magnitude and temperature dependence are significant in both fundamental and technological considerations. Recently, many extensive studies are going on in the semiconductor nanocrystals because they exhibit strong size dependent optical properties. These will be the key structural parameters in the fabrication of novel electronic nanodevices and nanocircuits. Semiconductor particles exhibit size dependent properties such as the scaling of the energy gap and corresponding change in the optical properties. CdSe is one of such materials, shows strong fluorescence which can be tuned according to the particle size. CdSe has been considered in many applications such as optoelectronic devices (Nazzal et al. 2003), light sensors (Bruchez et al. 1998), biological labels (Colvin et al. 1994), chemical libraries (Gaponik et al. 2002), etc. The nanopowder of CdSe provides excellent and unique properties which depend upon the shape and size of the nanostructures (Haram et al. 2001; Wang et al. 2004; Datta and Das 1990; Peng et al. 2000). Various methods such as hydrothermal, sol–gel approach, surfactant-assisted approach, etc. had been utilized for the synthesis of nanoparticles (Tang et al. 2003; Busbee et al. 2003). Synthesis, structural, and optical properties of CdSe nanoparticles have been reported (Dwivedi et al. 2011). In the present study, the main focus is on the electrical properties of pellets of nanoparticles of CdSe at different temperatures. The frequency dependence of dielectric constant, dielectric loss and ac conductivity was also investigated.
Materials and methods
Nanoparticles of CdSe were synthesized by solvothermal method. The chemicals such as cadmium nitrate and sodium selenite were used as the precursor materials to prepare CdSe nanoparticles. Cadmium chloride and sodium selenite in the molar ratio of 2:1 was dissolved in de-ionized water and stirred well by magnetic stirrer for 30 min. Hydrazine hydrate and ethylenediamine tetraacetic acid were used as complexing agents. Then the solution was centrifuged, filtered, and washed. Finally, the dark red product was collected by centrifugation and dried at 50 °C for 1 h. The dielectric measurements using HIOKI 3532-50 LCR HITESTER in the frequency range of 100 Hz and 1 MHz at various temperatures were carried out. The electrodes on either side with air-dying silver paste were made on the pelletized sample which behaves like a parallel plate capacitor. The dielectric studies were carried out at different temperatures and different frequencies. In this experiment, palletized nanoparticles using very high pressure have been used, so interfaces with this kind of large volume fraction in the nanosized samples must contain a great deal of defects including micro porosities, vacancies, vacancy clusters and dangling bonds. These defects can cause a change of positive and negative space-charge distributions in the interfaces. The pallets exposed to an external electric field, positive and negative charges on interfaces moves towards the negative and positive poles of electric field, respectively. Meanwhile, a great number of dipole moments are unavoidably formed after they have been trapped by defects. Consequently, space-charge polarization occurs in the interfaces of CdSe nanoparticles, which results in the much larger dielectric constant for CdSe nanoparticles. For conventional CdSe powders, it is impossible to observe space-charge polarization due to the smaller specific surface.
Results and discussion
X-ray diffraction analysis
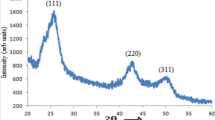
X-ray diffraction pattern of the as prepared CdSe nanoparticles is shown in Fig. 1. From the XRD pattern, it is clear from the broadening of diffraction peaks of that the particles crystallize at nanoscale range. The intensity of the peaks shows that the CdSe nanoparticles are of high crystalline and there is no trace of cubic phase. The peaks (1 0 0), (0 0 2), (1 0 1), and (1 0 3) are corresponding to the hexagonal phase. Generally, the (1 0 1) diffraction peak in hexagonal CdSe is strong and narrow, which may be attributed to the preferential growth along (1 0 1) plane of CdSe nanoparticles. From the experimental results, the average particle size of CdSe nanoparticles was calculated from the Debye–Scherrer formula,
where A is coherence length, β is the full-width at-half maximum (FWHM) of the diffraction peak, λ (1.5418 Å) is the wavelength of X-ray radiation, and θ is the angle of diffraction. The average particle size of the sample was found to be 50 nm which is derived from the FWHM of more intense peak using Scherrer formula.
SEM analysis
Scanning electron microscopy (SEM) was employed to analyze the morphology and the growth features of the aggregates of the as prepared CdSe nanoparticles. SEM images of as synthesized CdSe nanoparticles are shown in Fig. 2, which shows that the particles are well agglomerated. The pictures confirm the formation of CdSe nanoparticles. SEM images show that the surface of the spheres is moderately smooth, and a few degrees of aggregation of primary CdSe nanoparticles are also visualized.
Dielectric studies
The dielectric constant of an insulating material is the ratio of the capacitance of a parallel plate condenser measured with and without the dielectric material placed between the plates. The difference is, of course, due to the polarization of the dielectric. If the field applied to the condenser is time-dependent as in an alternating current, so is the polarization. However, because of the resistance to motion of the atoms in the dielectric, there is a delay between the changes in the field and changes in the polarization. This delay is often expressed as a phase difference or loss angle δ. The power factor is then defined as sin δ, and the dissipation factor as tan δ. The product of the dielectric constant and the power factor is called the loss factor and it is proportional to the energy absorbed per cycle by the dielectric from the field. The dielectric analysis is an important characteristic that can be used to fetch knowledge based on the electrical properties of a material medium as a function of temperature and frequency. Based on this analysis, the capability of the storing electric charges by the material and the capability of transferring the electric charge can be assessed. Dielectric properties are correlated with electro-optic property of the materials. Microelectronics industry needs low dielectric constant (εr) materials as an interlayer dielectric (Hatton et al. 2006). The dielectric constant is calculated using the formula,
where C is the capacitance (in F), the thickness, t (in m), the area, A (in m2), and εo the absolute permittivity in free space having a value of 8.854 × 10−12 Fm−1. From the graph (Fig. 3), it is clearly seen that the dielectric constant has high values in the low frequency regions for the CdSe nanoparticles. This is due to the existence of various types of polarization mechanisms like electronic, ionic, orientation and space-charge polarization (Mo et al. 1995). This may lead to large values of dielectric constant at low frequencies. Due to the application of an electric field, the space charges are moved and dipole moments are created and are called as space-charge polarization. In addition to this, these dipole moments are rotated by the field applied resulting in rotation polarization which is also contributing to the high values. Whenever there is an increase in the temperature, more dipoles are created and the value increases (Chawla et al. 2007). In the high frequency region, the charge carriers might have started to move and dielectric constant falls to a small value before the field reversal occurs. Figure 4 shows the variation of dielectric loss with respect to the frequency for various temperatures. It is quite obvious that the dielectric loss decreases with the increase in frequency for all temperatures which follows a similar trend with the decrease in the dielectric constant. This shows that the dielectric loss strongly rely on the frequency of the applied field. At high frequencies, the very low dielectric loss exhibited may be related to the purity of the nanomaterial, having negligible defects with good optical quality which could be useful in the design of optical devices (Barsoum 1977).
It is also observed that as the temperature increases, the dielectric constant also increases to a considerable value as seen in Fig. 5. The same trend is observed in the case of dielectric loss versus temperature as well given in Fig. 6. The larger value of the dielectric loss at lower frequency may be attributed to the space-charge polarization owing to charge lattice defect (Smyth 1965). The characteristic of low dielectric constant and dielectric loss with high frequency for a given sample suggests that the sample possesses enhanced optical quality with lesser defects and this parameter is of vital importance for various electro-optic devices and their applications (Austin and Mott 1969). The decrease in the value of dielectric constant with frequency is due to the fact that the frequency of electric charge carriers cannot follow the alternation of the ac electric field applied beyond a certain critical frequency (Ponpandian et al. 2002). The very low value of dielectric constant at higher frequencies is important for the fabrication of materials for ferroelectric, photonic and electro-optic devices. The value of dielectric constant at higher frequencies can be used to calculate Penn gap, Fermi energy and polarizability (Chauhan and Arora 2009).
Electrical conductivity studies
Electrical conductivity is a prominent factor which reveals the information about the transport phenomenon. CdSe with a Bohr exciton radius of 10 nm shows strong confinement effects. Recent work on electrical properties of semiconductor nanocrystalline has paid considerable attention towards the conduction mechanism (Joshi and Sehgal 2004). The ac conductivity plot of the pelletized form of CdSe nanoparticles is shown in Fig. 7. It is observed from the results that the ac conductivity increases with the increase in temperature, which shows the semiconducting nature of the sample. Due to the thermionic emission and tunnelling of charge carriers across the barrier, the conductivity increases with the temperature. Because of small size of the particles, the charge carriers reach the surface of the particles more and easily enabling the electron transfer by thermionic emission or tunnelling to enhance the conductivity (Mechant and Elbarum 1978). The ac conductivity of the nanoparticle sample, σac, can be calculated by the following relation:
where ε0 is permittivity in free space, εr is dielectric constant, f is the frequency and tan δ is the loss factor. There is a small increase in the electrical conductivity of the nanomaterial at the low frequency region for an increase in frequency and is the same for all temperatures. Conversely, at high frequencies especially in the KHz region, there is an abrupt increase in the conductivity and it is enormous at high temperatures which could be attributed to small polaron hopping (Barber et al. 2009).
In the case of nanoparticles, due to the small grain size and large grain boundaries, the electronic state close to Fermi level is localized. When the states are localized, the conduction occurs by hopping of carriers between occupied and unoccupied localized which depends on the density of state and the position of Fermi level. Mott (Elahi and Ghobadi 2008) established a dependence of conductivity with the temperature for such systems. The gradual decrease in the activation energy with the decrease in the temperature suggested that the conduction is by hopping of carriers among localized states (Johna et al. 2005). The high surface stress in the nanoparticles causes a lattice contraction which may not be symmetrical (Solliard and Flueli 1985; Gamarnik and Sidorin 1989). This may result in a lattice disorder which can be equivalent to a plastic deformation causing dislocations. It is not reasonable to argue that these dislocations may be considered to be one dimensional. It has been shown that, smaller the semiconductor particles, the greater the chance of the charge carriers to escape onto the semiconductor surface where electron transfers can occur (Fendler 1987). Experimental evidence strongly indicate that in small particles, the confinement of charge carriers perturb the band structure resulting in a series of discrete states in the conduction and valence bands and also causes an increase in the effective band gap. In case of pellets of small particles, the boundary between the particles must play an important role in determining the conductivity as in the case of polycrystalline semiconductor films (Kamins 1971). According to grain-boundary trapping theory, free carriers are trapped by the trapping states at the boundary causing a depletion of charges in the grain region nearest to the boundary (Seto 1975). Therefore, the region near the surface of the particle becomes depleted of charges causing a space charge which should establish an energy barrier between adjacent particles.
Conclusion
Nanoparticles of CdSe were synthesized using solvothermal method. The Powder X-ray analysis confirmed the nanoparticle size and the fundamental diffraction patterns of CdSe. The SEM reveals the morphology in the synthesized samples. The dielectric studies at different temperatures showed that the dielectric constant and dielectric loss have low values at higher frequencies and are independent of the temperature. The ac conductivity of the pellet form of CdSe nanoparticles were determined and observed that it increases with the increase in temperature. The enhancement in the conductivity and change in dielectric properties have been attributed to the special properties of nanometer-sized particles.
References
Austin IG, Mott NF (1969) Polarons in crystalline and non-crystalline materials. Adv Phys 18:41–102
Barber P, Balasubramanian S, Anguchamy Y (2009) Polymer composite and nanocomposite dielectric materials for pulse power energy storage. Materials 2:1697–1733
Barsoum M (1977) Fundamentals of ceramics. Mc Graw Hill, New York, p 543
Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281:2013–2016
Busbee BD, Obare SO, Murphy CJ (2003) An improved synthesis of high-aspect-ratio gold nanorods. Adv Mater 15:414–416
Chauhan KM, Arora SK (2009) Diamagnetic and photoabsorption characterisation of gel-grown cadmium oxalate single crystals. Cryst Res Technol 44:189–196
Chawla AK, Kaur D, Chandra R (2007) Structural and optical characterization of ZnO nanocrystalline films deposited by sputtering. Opt Mater 29:995–998
Colvin VL, Schlamp MC, Alivisatos AP (1994) Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature 370:354–357
Datta S, Das B (1990) Electronic analog of the electro-optic modulator. Appl Phys Lett 56:665–667
Dwivedi DK, Kumar V, Dubey M, Pathak HP (2011) Structural, electrical and optical investigations of CdSe nanoparticles. Chalcogenide Lett 8:521–527
Elahi M, Ghobadi N (2008) Structural, optical and electrical properties of CdSe nanocrystalline films. Iran Phys J 2:27–31
Fendler JH (1987) Atomic and molecular clusters in membrane mimetic chemistry. Chem Rev 87:877–899
Gamarnik MY, Sidorin YY (1989) Change of the unit cell parameters in highly dispersed gold, silver, and copper powders. Phys Stat Sol (b) 156:K1–K4
Gaponik N, Radtchenko IL, Sukhorukov GB, Rogach AL (2002) Toward encoding combinational libraries: charge-driven microencapsulation of semiconductor nanocrystals luminescing in the visible and near IR. Adv Mater 12:879–882
Haram SK, Quinn BM, Bard AJ (2001) Electrochemistry of CdS nanoparticles: a correlation between optical and electrochemical band gaps. J Am Chem Soc 123:8860–8861
Hatton BT, Landskron K, Hunks WJ, Bennett MR, Shukaris D, Perovic DD, Ozin GA (2006) Materials chemistry for low-k materials. Mater Today 9:22–31
Johna VS, Mahalingam T, Chu JP (2005) Synthesis and characterization of copper doped zinc telluride thin films. Solid State Electron 49:3–7
Joshi RK, Sehgal K (2004) Density of states near Fermi level in PbS nanoparticle films. Physica E 23:168–170
Kamins TI (1971) Hall mobility in chemically deposited polycrystalline silicon. J Appl Phys 42(4357):9
Mechant P, Elbarum C (1978) Anisotropic conductivity in plastically deformed ZnS. Solid State Commun 26:73–75
Mo CM, Zhang L, Wang G (1995) Characteristics of dielectric behavior in nanostructured materials. Nanostruct Mater 6:823–826
Nazzal AY, Qu L, Peng X, Xiao M (2003) Photoactivated CdSe nanocrystals as nanosensors for gases. Nano Lett 3:819–822
Peng XG, Manna L, Yang WD, Wickham J, Scher E, Kadavanich A, Alivisatos AP (2000) Shape control of CdSe nanocrystals. Nature 404:59–61
Ponpandian N, Balaya P, Narayanasamy A (2002) Electrical conductivity and dielectric behaviour of nanocrystalline NiFe2O4 spinel. J Phys Condens Matter 14:3221–3237
Seto JYW (1975) The electrical properties of polycrystalline silicon films. J Appl Phys 46(5247):8
Smyth CP (1965) Dielectric behaviour and structure. Mc Graw Hill, New York
Solliard C, Flueli M (1985) Surface stress and size effect on the lattice parameter in small particles of gold and platinum. Surf Sci 156:487–494
Tang KB, Qian YT, Zeng JH, Yang XG (2003) Solvothermal route to semiconductor nanowires. Adv Mater 15:448–450
Wang ZL, Kong XY, Ding Y, Gao P, Hughes WL, Yang R, Zhang Y (2004) Semiconducting and piezoelectric oxide nanostructures induced by polar surfaces. Adv Funct Mater 14:943–956
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Suresh, S., Arunseshan, C. Dielectric Properties of Cadmium Selenide (CdSe) Nanoparticles synthesized by solvothermal method. Appl Nanosci 4, 179–184 (2014). https://doi.org/10.1007/s13204-012-0186-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0186-5