Abstract
The present study reports the comparative analysis for the synthesis of zinc oxide nano particles by precipitation techniques using different zinc precursors. The synthesized nano particles were characterized by X-ray diffractometry (XRD), energy dispersive X-ray analysis and scanning electron microscopy (SEM) analysis for their sizes, shapes and arrangement. SEM has been studied for the samples before as well as after calcination to know the effect of temperature on structural behaviours. The XRD pattern shows the purity of synthesized zinc oxide nano particles and using Debye–Scherrer equation, the average crystal size of synthesized nanoparticles was calculated. The results have been discussed in the light of variation of morphological structures of different samples. Apart from this, the band gap energies of the synthesized particles have also been calculated from UV–visible spectrophotometric analysis, which is quite appreciable with the reported results.
Similar content being viewed by others
Introduction
Nano-sized particles have obtained much interest in the field of research due to their optimized properties and wide range of applications in different areas. Different metallic nano oxides show extensive utilizations in the fields such as optoelectronics (Wang et al. 2004; Lee et al. 2005) catalysis (Joshi et al. 2006), sensing (Cheng et al. 2004), solar cells, ultraviolet light emitter, piezoelectric device, chemical gas sensors (Eftekhari et al. 2006; Kim et al. (2007)) etc. Amongst all, zinc oxide nano particles have gained significant importance for both technical as well as fundamental applications. This oxide is a semiconductor material with a wide band gap (energy gap of 3.37 eV) and is used in electrical, photochemical, catalytic and optoelectronics applications. Due to electrostatic in nature, this can also be used in biomedical applications. In certain cases zinc oxide nanoparticles have neutral hydroxyl groups in their surface, which plays key role in charge behavior (Qu and Morais 1999, 2001). Again, due to large surface area, ZnO nanoparticles have great advantage in the field of catalytic reactions (Huang et al. 2006). Besides, these nanoparticles are used as glucose biosensors by making thin film with collagen base (Inbasekaran et al. 2014) and also it shows effective antimicrobial activities against pathogenic microorganisms (Sabir et al. 2014). As nano-fertilizers, ZnO colloidal sols are used to increase the yield and food crops growth (Selivanov et al. 2001; Raikova et al. 2006; Batsmanova et al. 2013). In addition, these nano structured oxides are also actively utilized in environmental science for water treatment (Shrishti et al. 2014). Therefore, nanostructured ZnO have acquired a special position in nano synthesis. Again in nano structures, the morphology of ZnO is an important parameter for the determination of physico-chemical properties of the crystals (Kawano and Imai 2008). Various studies related to different nano scale morphologies such as nano rods (Hu et al. 2003), nano spheres (Liu and Zeng 2004), nano whiskers (Li et al. 2004) have been reported earlier by many researchers. Meulenkamp (1998) reported about the crystal synthesis and growth of different zinc oxides. There are various techniques for the synthesis of these oxides and out of all, liquid phase synthesis process is the most feasible and versatile technique. Precipitation technique is one among these that involves zinc salts and hydroxides as precursors.
Present paper reports the synthesis of different zinc oxide nano particles by precipitation techniques using different zinc salts such as zinc sulphate, zinc acetate and zinc nitrate with aqueous sodium hydroxides. Efforts have been made to study comparatively for the synthesized ZnO nano particles for their change in morphological structures before and after calcinations.
Experimental section
All chemicals were obtained from Merck India and were of AR grade with >99% purity. These chemicals were used as received without any further purification. Deionized double distilled water was used throughout the experiment.
Synthesis of ZnO nano particles
Zinc oxide nano particles were synthesized by precipitation method using different zinc salts such as zinc acetate, zinc nitrate and zinc sulphate. To the aqueous solutions (0.1 M) of zinc salt, 0.2 M sodium hydroxide solution was added drop wise under constant stirring till the pH becomes 11. For the measurement of pH, SYSTRONICS make (Model: 335) digital pH meter was used. Initially the pH of the solution was 5.64 and it gradually increased with the addition of NaOH (for all precursors). Similarly, the formation of precipitations also increased gradually and finally dense white precipitations were obtained after continuous stirring up to 7 h to reach the pH value of 11. The precipitates were centrifuged and dried at 100 °C for 4 h. These samples were kept for overnight to dry and then ground to make fine powders of zinc oxide. Samples were calcined at 200, 400, 600 °C for 2 h followed by grinding to get fine particles. These were used for different characterization.

Structural and optical characterization
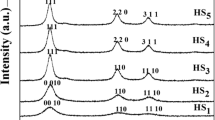
All the synthesized nano particles were characterized for their shapes, sizes and arrangement from XRD, EDXA and SEM analysis. The X-ray diffraction spectra of ZnO nano particles were taken using RIGAKU smart lab X-ray Diffractometer using CuKα radiation λ = 1.5405 Å and X-rays generator operating at 40 kv. The scanning range was maintained within 20°–100° with the scanning speed of 5° min−1. The XRD Patterns were presented in Fig. 1. The elemental composition of ZnO nano particles from EDXA and SEM analysis for shape and structures have been carried out using GEMINI ULTRA 55 instruments. The EDX images for all the samples have been presented in Fig. 2 and the details of elemental composition of zinc and oxygen are listed in Table 1. The SEM morphologies for the samples before and after calcinations at different resolutions and temperatures are shown in Figs. 3, 4 and 5.
a SEM morphologies of ZnO NPs from zinc sulphate before calcination (mixture of both grains and rods morphologies), b SEM morphologies of ZnO NPs from zinc sulphate after calcination with high resolution only for rods, c SEM morphologies of ZnO NPs from zinc sulphate after calcination with high resolution only for grains, d SEM morphologies of ZnO NPs from zinc sulphate after calcination at 200 °C, e SEM morphologies of ZnO NPs from zinc sulphate after calcination at 400 °C, f SEM morphologies of ZnO NPs from zinc sulphate after calcination at 600 °C
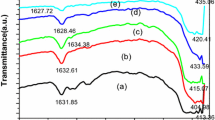
Besides, the UV–visible spectroscopic measurements were carried out at room temperature using ELICO made SL-159 UV–visible spectrophotometer in the range 300–800 nm to evaluate the band gap energy by extending the intersection peaks in Fig. 6.
Results and discussion
Structural analysis
The XRD patterns of zinc oxide nanoparticles show well defined peaks located at Bragg angles (2θ) = 31.72, 34.39 and 36.23 (for zinc acetate salt), whereas for zinc nitrate, the peaks were located at (2θ) = 31.70, 34.37 and 36.23. Similarly, for zinc sulphate precursor, the well-defined peaks were located at (2θ) = 31.75, 34.35 and 36.23. All these characteristic peaks are of higher in intensity corresponding to planes having miller indices (100), (002) and (101), respectively, and indicates that the products obtained are pure and in good crystalline nature. No peaks corresponding to impurities were detected. These data again gives the information about the formation of hexagonal wurtzite structure for all ZnO nanoparticles (Ref:JCPDS file no. 36–1451). The average crystallite sizes (τ) were calculated using Debye–Scherrer equation as below (He et al. 2008). The average crystal sizes for different samples obtained from the relation in nano scale have been presented in Table 1:
where K is Scherrer constant and the crystallite shape factor, λ represents the wavelength of X-ray source 1.5405 Å used in XRD, β is full width at half maximum of diffraction peak, and θ is the Bragg angle of intense peak. From the data of obtained diffraction pattern for this method, the calculated average crystallite size was found to be 23, 19 and 37 nm for the different zinc salts of acetate, nitrate and sulphate, respectively. The EDX spectrums show good agreement with the results of XRD analysis. However, nano particles synthesized from the nitrates contain only zinc oxide by weight percentage (100%) without any other trace element or impurities. The SEM images for three different synthesized ZnO nanoparticles have been taken at different resolutions and temperatures. For zinc acetate precursor, the nano particles were found to be well defined triangular shaped with nano meter sized in the range of 97–174 nm and also few particles are found to be in bunches due to agglomeration. There is no much difference in structural arrangement on applying temperature as observed from SEM analysis after calcinations for 200, 400 and 600 °C. Similarly, for zinc nitrate precursor, irregular and triangular shaped nano particles were observed for both before and after calcinations with the particles sizes 52–93 nm. Whereas in case of zinc sulphate precursor, the nano particles before calcinations were found to be well defined rod shaped as well as grain shaped structures (with flower type modification). There are slight changes in structures at 200 °C, where both rods and flakes were observed. But the shapes of all nano particles were changed to flakes or petals types at 400 °C. However, there is no much variations at 600 °C. Review of literature shows that even from zinc nitrate (Hasanpoor et al. 2015; Kataria et al. 2016) and zinc acetate (Khan et al. 2014) precursors, flower shaped ZnO nano particles have obtained. However, Osman et al. reported for flakes type nano particles from zinc acetate precursor (Osman and Mustafa 2015).
The absorption peaks from UV–visible spectroscopy were observed in between 356 and 375 nm for all samples. The absorption peak is probably related to the electronic transition taking place from valence band to the conduction band due to quantum size of particles (Yin et al. 2005). the optical band gap energy, E g of synthesized ZnO nanoparticles can be calculated by extrapolating the linear portion of the absorbance spectrum to zero to get λ and using the following equation (Kannaki et al. 2012, Dharma and Pisal 2009):
where h is the Plank’s constant and ν is the frequency (ν = c/λ). In the present investigation, the E g for all the synthesized particles are found to be in the range 3.323–3.4898 eV (Table 1). As per literature (Harun et al. 2016), the E g for ZnO was reported to be 3.37 eV.
Conclusion
In the present investigation, ZnO nanoparticles were synthesized successfully using precipitation technique with three different zinc precursors separately. XRD analysis confirmed the synthesis of highly pure ZnO nanoparticles. And data obtained from EDX analysis is a supplement to the above findings. The most interesting observation that was obtained in this present investigation is that the particles synthesized from sulphate precursors showed very nicely organized rods and grains morphology arranged like flowers before calcinations but the morphology changed after calcination at 400 °C to only flakes type. However, the morphology did not alter even after calcinations for the nano particles synthesized from other two precursors such as zinc acetate and zinc nitrate. The band gap energy for all the nano particles are found to be in the range 3.313–3.484 eV, which is very near to the literature value of 3.37 eV. The major objective of this investigation is to extend the study by utilizing these synthesized nano particles for their efficiencies in different applications such as in the field of mechanical and environmental sciences.
References
Batsmanova LM, Gonchar LM, Taran NY, Okanenko AA (2013) Using a colloidal solution of metal nanoparticles as micronutrient fertiliser for cereals. Proc Int Conf Nanomater 2:2
Cheng XL, Zhao H, Huo LH, Gao S, Zhao JG (2004) ZnO nanoparticulate thin film: preparation, characterization and gas-sensing property. Sens Actuators B 102:248–252
Dharma J, Pisal A (2009) Simple method of measuring the band gap energy value of TiO2 in the powder using UV/vis/NIR spectrometer. Perkin Elmer, Inc., Shelton, pp 1–4
Eftekhari A, Molaei F, Arami H (2006) Flower-like bundles of ZnO nanosheets as an intermediate between hollow nanosphere and nanoparticles. Mater Sci Eng A 437(2):446–450
Harun K, Mansor N, Ahmad ZA, Mohamad AA (2016) Electronic properties of ZnO nanoparticles synthesized by Sol-gel method: a LDA+U calculation and experimental study. Procedia Chem 19:125–132
Hasanpoor M, Aliofkhazraei M, Delavari H (2015) Microwave-assisted synthesisof zinc oxide nanoparticles. Procedia Mater Sci 11:320–325
He C, Sasaki T, Shimizu Y, Koshizaki N (2008) Synthesis of ZnO nanoparticles using nanosecond pulsed laser ablation in aqueous media and their self-assembly towards spindle-like ZnO aggregates. App Surface Sci 254:2196–2202
Hu Z, Oskam G, Searson PC (2003) Influence of solvent on the growth of ZnO nanoparticles. J Colloid Interface Sci 263:454–460
Huang YH, Zang Y, Liu L, Fan SS, Wei Y, He (2006) Controlled synthesis and field emission properties of ZnO nanostructures with different morphologies. J Nanosci Nanotechnol 6:787–790
Inbasekaran S, Senthil R, Ramamurthy G, Sastry TP (2014) International journal of innovative research in science. Eng Technol 3(1):8601–8606
Joshi SS, Patil PR, Naimase MS, Bakare PP (2006) Role of ligands in the formation, phase stabilization, structural and magnetic properties of α-Fe2O3 nanoparticles. J Nanopart Res 5:635–643
Kannaki K, Ramesh PS, Geetha D (2012) Hydrothermal synthesis of CuO nanostructure and their characterizations. Int J Sci Eng Res 3(9):1–4
Kataria N, Garg VK, Jain M, Kadirvelu K (2016) Preparation, characterization and potential use of flower shaped zinc oxide nanoparticles (ZON) for the adsorption of Victoria Blue B dye from aqueous solution. Adv Powder Technol 27(4):1180–1188
Kawano T, Imai H (2008) A simple preparation technique for shape-controlled zinc oxide nanoparticles: formation of narrow size-distributed nanorods using seeds in aqueous solutions. Colloids Surf A 319:130–135
Khan MF, Hameedullah M, Ansari AH, Ahmad E, Lohani MB, Khan RH, Alam MM, Khan W, Husain FM, Ahmed I (2014) Flower-shaped ZnO nanoparticles synthesized by a novel approach at near-room temperatures with antibacterial and antifungal properties. Int J Nanomed 9:853–864
Kim KD, Choi DW, Choa YH, Kim HT (2007) Optimization of parameters for the synthesis of zinc oxide nanoparticles by Taguchi robust design method. Colloids Surf A 311:170–173
Lee SY, Shim ES, Kang HS, Pang SS (2005) Fabrication of ZnO thin film diode using laser annealing. Thin Solid Films 437:31–34
Li P, Wei Y, Liu H, Wang X (2004) A simple low-temperature growth of ZnO nanowhiskers directly from aqueous solution containing Zn(OH) 2−4 ions. Chem Commun 24:2856–2857
Liu B, Zeng HC (2004) Room temperature solution synthesis of monodispersed single-crystalline ZnO nanorods and derived hierarchical nanostructures. Langmuir 20:4196–4204
Meulenkamp AE (1998) Synthesis and growth of ZnO nano particles. J Phys Chem B 102:5566–5572
Osman DAM, Mustafa MA (2015) Synthesis and characterization of zinc oxide nanoparticles using zinc acetate dihydrate and sodium hydroxide. J Nanosci Nanoeng 1(4):248–251
Qu F, Morais PC (1999) Energy levels in metal oxide semiconductor quantum dots in water-based colloids. J Chem Phys 111:8588–8594
Qu F, Morais PC (2001) The pH dependence of the surface charge density in oxide-based semiconductor nanoparticles immersed in aqueous solution. IEEE Trans Magn 37:2654–2656
Raikova OP, Panichkin LA, Raikova NN (2006) Studies on the effect of ultrafine metal powders produced by different methods on plant growth and development. In: Nanotechnologies and information technologies in the 21st century in Proceedings of the International Scientific and Practical Conference. 108
Sabir S, Arshad M, Chaudhari SK (2014) Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications. Sci World J 8. doi:10.1155/2014/925494
Selivanov VN, Zorin EV, Perspekt (2001) Sustained Action of ultrafine metal powders on seeds of grain crops. Materialy 4:66–69
Shrishti A, Kumar PS, Monika S (2014) Solar light assisted nano ZnO photo catalytic mineralization—the green technique for the degradation of detergents. Int J Chem Pharma Anal 1(3):141–147
Wang ZL, Kong XY, Ding Y, Gao P, Hughes (2004) Semiconducting and piezoelectric oxide nanostructures induced by polar surfaces. Adv Funct Mater 14:943–956
Yin M, Wu CK, Lou Y, Burda C, Koberstein JT, Zhu Y, O’Brien S (2005) Copper oxide nanocrystals. J Am Chem Soc 127:9506–9511
Acknowledgements
Susmita Kamila is thankful to SERB-DST, for the award of Young Scientist under Start-up-Research Grant. Dr. B. M. Satish, Principal, East Point College of Engineering and Technology is gratefully acknowledged for his constant encouragement and support for the work. One of the authors (VRV) is grateful to Visvesvaraya Technological University (VTU), Belagavi, Karnataka for his Ph.D program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Venu Gopal, V.R., Kamila, S. Effect of temperature on the morphology of ZnO nanoparticles: a comparative study. Appl Nanosci 7, 75–82 (2017). https://doi.org/10.1007/s13204-017-0553-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-017-0553-3