Abstract
Conversion of lignocellulosic biomass into monomeric carbohydrates is economically beneficial and suitable for sustainable production of biofuels. Hydrolysis of lignocellulosic biomass using high acid concentration results in decomposition of sugars into fermentative inhibitors. Thus, the main aim of this work was to investigate the optimum hydrolysis conditions for sorghum brown midrib IS11861 biomass to maximize the pentose sugars yield with minimized levels of fermentative inhibitors at low acid concentrations. Process parameters investigated include sulfuric acid concentration (0.2–1 M), reaction time (30–120 min) and temperature (80–121 °C). At the optimum condition (0.2 M sulfuric acid, 121 °C and 120 min), 97.6% of hemicellulose was converted into xylobiose (18.02 mg/g), xylose (225.2 mg/g), arabinose (20.2 mg/g) with low concentration of furfural (4.6 mg/g). Furthermore, the process parameters were statistically optimized using response surface methodology based on central composite design. Due to the presence of low concentration of fermentative inhibitors, 78.6 and 82.8% of theoretical ethanol yield were attained during the fermentation of non-detoxified and detoxified hydrolyzates, respectively, using Pichia stipitis 3498 wild strain, in a techno-economical way.
Similar content being viewed by others
Introduction
The energy consumption is expected to continue increasing rapidly owing to high economic growth, increasing populations and ongoing industrialization which has led to depletion of fossil fuels. Hence, the production of alternative energy from renewable resources is very essential to fulfill the future generation requirements. The interest of modern research has been switched from food-based ethanol (first-generation biofuels from sweet sorghum grains, sugarcane and corn) to non-food-based ethanol (second-generation biofuels from lignocellulosic biomass) (Naik et al. 2010).
Inedible agricultural lignocellulosic materials such as sorghum biomass, corn stover, rice husk and wheat straw are abundantly available on the earth. Among them, sorghum (Sorghum bicolor (L) Monech) biomass is considered one of the most promising feedstock for the production of second-generation biofuels. The inherent genetic diversity and tolerance to heat and drought conditions of sorghum enables to target the development of new traits via genetic modifications (GM), thereby enhancing the palatability and reducing the lignin content of sorghum (Rao et al. 2009). Therefore, development of brown midrib (bmr) sorghum varieties has become a significant achievement for the bioenergy applications (Chen and Dixon 2007; Vermerris et al. 2007; Dien et al. 2009) and forage digestibility (Barriere et al. 2003; Guo et al. 2001; Jung and Allen 1995; Vogel and Jung 2001).
Hetero-polymeric structure of lignocellulosic material is made up of cellulose, hemicellulose and lignin (Rowell et al. 2005). Hemicellulose and cellulose are the polymeric carbohydrates which consist of pentose (xylose and arabinose) and hexose (glucose) sugars, respectively. Fractionation and hydrolysis of these polymeric carbohydrates is important for commercialization of bioethanol production process. Therefore, pretreatment is an essential step to disrupt the complex network of lignocellulosic material to hydrolyze the hemicellulose and alter the cellulose structure to make it more accessible to the enzymatic hydrolysis. Several pretreatment methods have been developed to depolymerize the lignocellulosic materials which include steam explosion, acid hydrolysis and hot water pretreatment (Mosier et al. 2005). However, most of these pretreatment methods require high-energy input, high temperature and high acid strength, which often result in formation of toxic compounds such as furfural from pentose sugars and 5-hydroxyl methyl furfural (5-HMF) from glucose (Zhao et al. 2007). These are the potential toxic compounds which inhibit microbial growth and lead to a low yield of ethanol during the prehydrolyzate fermentation. Over the years, different methods have been developed to overcome the inhibition effect of microbial growth which includes, ion exchange chromatography (Chandel et al. 2007), a prior adaptation of microorganisms to prehydrolyzate (Huang et al. 2009) and genetic modifications in microorganisms through UV mutations (Rakesh et al. 2012). These methods are tedious and proper skills are required for development. Even though overliming is a well-established process for detoxification of prehydrolyzates, a major disadvantage is sugar loss, and it is not an effective way to reduce the toxicity caused by organic acids (Palmqvist and Hahn-Hagerdal 2000). Overliming followed by activated charcoal adsorption increases the process cost and the sugars loss was higher than overliming treatment (Jing Ping et al. 2011). From the aforementioned literature, it was suggested that the development of pretreatment conditions for maximization of pentose sugars yield along with the minimized level of fermentative inhibitors from sorghum biomass would be challenging.
Therefore, the present study has been focused on the development of an effective dilute acid pretreatment process which maximizes the hemicellulose hydrolysis to achieve the high yield of pentose sugars with the less amounts of fermentative inhibitors. In addition, response surface methodology (RSM) was employed to determine the effects of various pretreatment parameters on pentose sugars yield and furfural formation. Fermentation of prehydrolyzate was carried out for bioethanol production to support developed optimum acid pretreatment condition.
Materials and methods
Biomass source
Sorghum (Sorghum bicolor (L) Moench) brown midrib (bmr) IS11861 was procured from the International Crop Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, Hyderabad, Telangana, India. The dried biomass was milled and sieved to achieve the particle size of 300–150 µm and subjected to oven drying at 45 ± 3 °C for 48 h as described in National Renewable Energy Laboratory (NREL) protocol (Hames et al. 2008).
Composition analysis of biomass
The chemical composition of SBMR IS11861 biomass was analyzed according to the standard NREL laboratory analytical procedure (LAP). Biomass was subjected to Soxhlet extraction with water (12 h) and ethanol (8 h). This two-stage extraction process was performed to remove extractives such as fertilizers, nitrites/nitrates, chlorophyll, waxes and proteins (Sluiter et al. 2005). The water and ethanol extractives were concentrated using rotary evaporator® (Buchi, R-210, Switzerland) under reduced pressure, and then oven dried at 105 °C to measure the overall extractives weight. The extractive-free biomass was oven dried for 48 h at 45 ± 3 °C and then, structural carbohydrates and lignin contents were analyzed according to NREL procedure (Sluiter et al. 2011).
Pretreatment parameters
The biomass to liquid ratio of 1:20 (w/v) was mixed with different dilute sulfuric acid concentrations (M), viz., 0.2, 0.4, 0.6, 0.8 and 1 M and each of them was hydrolyzed for 2 h at different temperatures such as 80 ± 2, 100 ± 2 and 121 ± 1 °C using water bath (Lauda, Labtech, India) and autoclave, respectively. At every 30 min time interval, stop the reaction and allow it to cool at room temperature. From the reaction mixture, 100 µl of a sample was withdrawn and analyzed using high-performance liquid chromatography (HPLC) for the quantification of sugars and fermentative inhibitors.
Experimental design
According to the preliminary biomass pretreatment results, the release of pentose sugars and furfural formation were statistically optimized through central composite design (CCD) by Design expert software® trial version 10 (Stat-Ease, inc., Minneapolis, MN, USA). From the CCD model, 20 distinct experimental conditions were obtained which are summarized in Table 1. From the preliminary results, it was found that at 120 °C, 0.2 M and 120 min the pentose sugars yield was higher than other conditions. Furthermore, we have extended the pretreatment variable conditions to somewhat higher level to check whether the sugars yield will increase or decrease. Considered independent pretreatment variables for RSM were: temperature (X 1) = 100, 120, 140 °C; reaction time (X 2) = 90, 120, 150 min; H2SO4 concentration (X 3) = 0.1, 0.2, 0.3 M. The investigated response variables were pentose sugars and furfural in the prehydrolyzates.
From the experimental results, the obtained values of response variables were subjected to a regression analysis to find out the interaction effect of factors using the least square method (Djioleu and Carrier 2016). The common form of second-order polynomial obtained from the regression analysis is depicted in Eq. 1. This second-order polynomial was used to evaluate the effect of independent variables on the response which was further analyzed to obtain the optimum pretreatment conditions (Tan et al. 2011). Models and regression coefficients were authenticated with an analysis of variance (ANOVA). The significance for any statistical result was established for P value <0.05.
where Y is the response (pentose sugars and furfural yield), β 0 is the constant coefficient β i is the ith linear coefficient, β ii is the quadratic coefficient, and β ij is the ijth interaction coefficient. X i and X j are independent variables. CCD consists of 2 k factorial points, 2k axial points (±α), and six central points, where k is the number of independent variables.
Production of bioethanol from prehydrolyzate
Microorganism
Pichia stipitis NCIM 3497 (Same as CBS 6577) strain was procured from the National Collection of Industrial Microorganisms (NCIM) Pune, India. P. stipitis was subcultured on YEPX medium containing (g/L): 10, yeast extract; 20, peptone; 20, xylose; 20, agar and incubated at 30 °C for 48 h. Colonies from the plates were transferred into filter-sterilized liquid broth containing (g/L): urea—2.27, yeast nitrogen base—1.7, peptone—6.56, and xylose—20. After 18 h incubation time, the cells were harvested by centrifugation at 5000 rpm for 5 min and re-suspended in sterile distilled water to a final concentration of 40 g dry cells/L (serves as inocula).
Fermentation of prehydrolyzate
Fermentation studies were performed using both non-detoxified and detoxified hydrolyzates. For the preparation of non-detoxified and detoxified hydrolyzates, the hydrolyzate was first heated to 50 °C and held at this desired temperature for 15 min. This was followed by the slow addition of calcium hydroxide [Ca(OH)2] to reach pH of the hydrolyzate to 7 and 10 for neutralization and detoxification, respectively. Agitation was then carried out for 30 min. The calcium sulfate (CaSO4) sludge and the liquid were next separated by filtration. Finally, the filtered hydrolyzates’ pH was adjusted to cultivation pH (6) of Pichia stipitis with 10N H2SO4. Prior to the fermentation, 50% of liquid was separated from hydrolyzate without affecting the sugars by rotary evaporator. This process eventually increases the sugars concentration up to onefold in the remaining hydrolyzate.
Fermentation experiments were performed in sterile 50-mL Erlenmeyer flasks containing 20 mL of filter-sterilized production medium which includes 0.4 mL of 50X nutrient solution (prepared by dissolving 2.27 g of urea, 1.7 g of yeast nitrogen base and 6.56 g of peptone in 20 mL of water), 0.6 mL of 1 M phosphate buffer (KH2PO4/NaOH, pH 6) and 0.5 mL of inocula which give the initial cell concentration of 2 g/L. Medium pH was adjusted to 6 with 10N H2SO4 and all these experiments were performed at 30 °C for 72 h.
HPLC analysis for the quantification of sugars and fermentative inhibitors
Sugars (glucose, xylose, arabinose), fermentative inhibitors (5-HMF, furfural, formic acid, acetic acid) and ethanol concentrations were analyzed using HPLC. The separation system was equipped with a solvent delivery system (210), refractive index (RI) detector (355) (Varian, The Netherlands) and Meta Carb-87H carbohydrate column (300 × 6.5 particle size 8 µm). The column temperature was maintained at 60 °C and 9 mM sulfuric acid was used as an eluent at 0.5 mL/min flow rate. HPLC peaks were identified by authentic standards based on specific retention time of each compounds.
Results and discussion
Compositional analysis
The composition of structural carbohydrates and lignin contents of biomass are shown in Table 2. SBMR IS11861 biomass contains 34.8% of cellulose, 29.7% of hemicellulose and 14.3% of lignin. Cellulose was found to be a major carbohydrate polymer present in the sorghum biomass. The chemical composition of hemicellulose varies with species to species and according to the literature, wheat straw and grasses contain xylan, arabinan and galactan (Grohmann et al. 1984; Torget et al. 1990), while other hardwood and softwood biomass contains one more component, i.e., mannan in their hemicellulose composition (Torget et al. 1990; Brigham et al. 1996). The results of the present study revealed that hemicellulose composition of SBMR IS11861 biomass mainly consists of xylan, arabinan, glucuronic acids and acetyl groups.
Effect of pretreatment parameters on sugar yields
Xylobiose, glucose, xylose and arabinose have been found to be the principal sugars during the dilute acid pretreatment of SBMR IS11861 biomass. Apart from reducing sugar formation, pretreatment reaction can also produce fermentative inhibitors, such as 5-HMF, furfural, formic acid and acetic acid. Reaction temperature, time and acid concentration are the key parameters which affect the sugars release and their degradation. The concentration of sugars was calculated based on the following equation (Eq. 2).
Conversion of pentose sugars and their degradation products
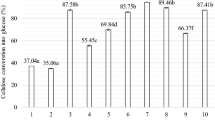
During the dilute acid pretreatment, conversion of hemicellulose into monomeric sugars occurred in two steps which include (1) cleavage of covalently bonded acetyl groups form xylan backbone and (2) splitting of glycosidic linkages between xylose and arabinose units (Kamireddy et al. 2013). Sulfuric acid acts as a catalyst to breakdown the glycosidic linkages present in polymeric carbohydrates. It can be observed that the catalytic effect of sulfuric acid increased with an increase in the temperature. From Fig. 1, it can be observed that the hydrolysis of hemicellulose increases with the increase in the reaction temperature (80–121 °C). At 80 °C, 0.2 M and 30 min reaction time, 8.8 mg of xylose and 4.08 mg of arabinose were attained. Further increase in temperature (80–121 °C) and time (30–120 min) at 0.2 M acid concentration, xylose and arabinose concentrations were increased significantly to 225.2 and 20.2 mg, respectively. It is also evident from Fig. 2a and b, with an increase in sulfuric acid concentration at 121 °C, xylose and arabinose concentration decreases which could be due to their decomposition. It has been reported that xylose can be easily degraded to furfural at a temperature higher than 120 °C (Liu et al. 2012). In the present study, furfural concentration was increased with increasing sulfuric acid concentration and reaction time at 121 °C (Fig. 2c). This phenomenon indicates cylcodehydration of xylose to form furfural, i.e., removal of three water molecules from xylose is responsible for the furfural formation (Kamireddy et al. 2013). Similarly, arabinose being a geometrical isomer of xylose similar results of furfural formation can be expected (Garrett and Dvorchik 1969). Further, furfural decomposes to form formic acid with an increase in the pretreatment severity (Niu et al. 2015) which is shown in Fig. 2d. The detailed reaction pathway for the conversion of hemicellulose to pentose sugars and their decomposition products are shown in Fig. 3.
The optimized condition for hemicellulose hydrolysis has been determined as temperature = 121 °C, time = 120 min and sulfuric acid = 0.2 M. As a result, 97.6% of hemicellulose is significantly converted into 18.02 mg of xylobiose, 225.2 mg of xylose and 20.2 mg of arabinose with 4.6 mg (or 0.23 g/L) of furfural and 2.3 mg of formic acid. Vancov and McIntosh (2012) reported that approximately 55% of hemicellulose in the sorghum bicolor straw has been hydrolyzed to yield 150 mg/g of xylose at 121 °C for 60 min in the presence of 2% sulfuric acid. In another study, corn stover is pretreated at 200 °C for 14.3 min, releasing approximately 77.3% of xylose and its oligomers (Zhang et al. 2015). Kamireddy et al. (2013) studied the dilute acid hydrolysis of sorghum brown midrib (SBMR) and sorghum non-brown midrib (SNBMR) in a batch reactor at a temperature ranging from 150 to 160 °C, 1–2% sulfuric acid concentration and reaction time of 10–20 min. According to Kamireddy et al. (2013), xylose yield of 95 and 91% was observed in SNBMR and SBMR, respectively, with a varying furfural concentration of 0.75–3.4 g/L for SBMR and 0.68–3.81 g/L for SNBMR. So far, compared to the literature, the method used in the present study is considered as an efficient method for hemicellulose hydrolysis of lignocellulosic biomass, which yields maximum xylose and arabinose with minimum concentration of sugar-degraded products. Furthermore, the obtained xylobiose, xylose and furfural concentrations at selected pretreatment parameters are shown in Fig. A1 (supporting information). In addition to this, acetic acid is one of the most encountered by-products during the acid pretreatment which is derived from the hemicellulose constituent of acetylated xylan (Liu et al. 2012). The formation of acetic acid was initiated at the beginning of hydrolysis reaction is shown in Fig. A2.
Conversion of hexose sugars and its degradation products
Along with hemicellulose hydrolysis, acid pretreatment can also hydrolyze the cellulose polymer of sorghum biomass to produce glucose units. During the acid pretreatment, cellulose hydrolysis was found to be comparatively lower than that of hemicellulose. From Fig. 4a, it can be seen that the low levels of glucose yield is obtained during the sorghum biomass hydrolysis. In general, two types of cellulose are present in the lignocellulosic biomass, i.e., amorphous and crystalline cellulose. The percentage of crystalline cellulose is higher than amorphous cellulose. The crystalline cellulose is thermodynamically stable due to the presence of strong intra- and inter-linked hydrogen bonds between the glucan chains (Krassig et al. 2004). This might be a reason for the low concentration of glucose yield during the acid pretreatment. Apart from that some fraction of glucose and 5-HMF were also observed at 121 °C. 5-HMF is a dehydration product of glucose. With the increase in reaction time at constant 0.2 M acid concentration, 5-HMF formation found to increase. Further, with increase in acid concentration and reaction time, 5-HMF concentration decreased (Fig. 4b); this could be due to the decomposition of 5-HMF into levulinic acid and formic acid (Qi et al. 2014). From the above discussion, it was clear that all the three process parameters, viz., temperature, acid concentration, and time have a significant influence on the hydrolysis. Nonetheless, the combination of optimum process parameters yields less concentration of 5-HMF (5.1 mg) at optimum pretreatment condition.
Carbohydrate analysis of pretreatment-derived residual biomass
After the hydrolysis, solid and liquid fractions were separated through vacuum filtration using a 0.2-µm nylon membrane. The hydrolyzed biomass was washed with distilled water to attain a neutral pH and then dried at 45 ± 3 °C for 48 h. It was observed that 51% of SBMR IS11861 biomass was significantly hydrolyzed at optimum pretreatment condition. The residual biomass compositional analysis was carried out according to the modified NREL protocol (Sluiter et al. 2011). It was found that the residual biomass (490 mg) contains 275.3 mg of cellulose, 6.86 mg of xylan and 120 mg of lignin. However, per gram basis, enriched content of cellulose (56.2%) and a very low amount of xylan (1.4%) were present in the acid pretreated biomass (Table 2).
Statistical impact of pretreatment parameters on pentose sugars release and furfural formation
Response surface methodology (RSM) is a statistical approach to analyze the importance of each individual pretreatment parameter and their interactions on response variables. RSM has several advantages such as consumes less time, inexpensive, and can investigate the various numbers of factors at a time with a minimum number of experiments. Central composite design (CCD) is one of the most popular models to optimize the independent variables. According to the CCD model, each and every factor of the experiments is simultaneously varied with all possible combinations for the determination of variable interaction effects on the response. In the present study, CCD model has been employed and executed to determine the influence of pretreatment temperature, time and acid concentration on pentose sugars and furfural formation. Such analysis could be extremely useful in the conversion of lignocellulosic biomass into fermentable sugars and their degradation for further production of biofuels and value-added products.
ANOVA analysis
A quadratic model has been developed from the experimental data for each response (i.e., pentose sugars and furfural). Analysis of variance (ANOVA) demonstrated that the developed quadratic model for pentose sugars and furfural is the most significant, as their P values are less than 0.05. The individual pretreatment parameters and their interaction effects on response variables were determined by the regression coefficients (R 2) as 0.94 and 0.95 for pentose sugars and furfural, respectively. The regression model equation resulting from ANOVA analysis in terms of coded factors for response variables is given in the following equations:
From the results (shown in supplementary information Table A1, A2), pretreatment temperature and acid concentration showed significant effect on the response variables (pentose sugars and furfural), whereas the reaction time showed less significance on both the responses. It was also noticed that there is a significant interaction effect between pretreatment temperature and time on pentose sugars yield.
3D response surface and contour plots illustrated the interaction effect of experimental independent variables on pentose sugars and furfural yield. The significant effect on the response variable can be observed by varying two factors at a time and keeping the other factor at a constant level. These plots are extremely important to investigate and understand the interaction effects between the two factors on the response variables. Figure 5a shows the interaction between temperature and acid concentration, in which the maximum pentose sugar yield increases at the center of the region (zero level). On the other hand, with an increase in the acid concentration at high temperature the pentose sugars yield decreases. Figure 5b and c indicates the interaction between pretreatment temperature and acid concentration with time on pentose sugars yield, respectively. The concentration of pentose sugars was increased at a fixed zero level of temperature and time. Varying the affecting variables such as temperature and time levels at a constant acid concentration leads to decrease the pentose sugars concentration. This could be due to the formation of pentose sugar-degradation product such as furfural.
The effect of temperature, time and acid concentrations on the formation of furfural are also shown in 3D response surface plots (Fig. 6). The interaction between time with the temperature (Fig. 6a) and acid concentration with temperature (Fig. 6b) continuously enhances the furfural concentration. On the other hand, increasing and then slightly decreasing trend was observed in the furfural concentration during the interaction between acid concentration and time in the surface plot Fig. 6c. Due to the prolonged pretreatment time, furfural can be decomposed into formic acid.
Validation of predicted response at the optimum condition
From Table 1, the optimum condition for maximum pentose sugars yield along with the less concentration of furfural was obtained at T = 120.3 °C, t = 102.38 min, and C = 0.215 M, whereas the predicted response of pentose sugars and furfural yield was 241.2 and 4.57 mg/g, respectively. To validate the predicted optimum condition responses, additional experiments were performed to examine the suitability of the model equation. From the experimental study, the pentose sugars and furfural concentrations were obtained as 244.7 and 4.66 mg/g, respectively. Hence, the predicted model is in close agreement with the pentose sugars and furfural concentrations. The results of current study validated that this model can effectively be applied on the hemicellulose hydrolysis of sorghum biomass for the production of maximum pentose sugars with low concentration of furfural. Prehydrolyzate containing a high concentration of pentose sugars (especially xylose) and the least concentration of fermentative inhibitor (furfural) may enhance the fermentation efficiency during the bio-based products production. Such analysis could be useful for designing a lignocellulosic biomass conversion process into fermentable sugars for the bio-refinery platform in techno-economical way.
Fermentation of non-detoxified and detoxified hydrolyzates
The fermentation results of both non-detoxified and detoxified hydrolyzates using P. stipitis NCIM 3497 are depicted in Table 3. As compared to the non-detoxified hydrolyzate, the highest ethanol yield (0.42 ± 0.01 gp/gs) and ethanol conversion (82.8%) were found in detoxified hydrolyzate, whereas the ethanol yield of 0.40 gp/gs, and 78.6% ethanol conversion was observed in the non-detoxified hydrolyzate. In addition to this, decreased ethanol productivity (0.32 ± 0.01 g/L/h) and the prolonged fermentation time (36 h) were observed for maximum ethanol production from the non-detoxified hydrolyzate. In the case of detoxified hydrolyzate fermentation, the maximum ethanol production was observed at 30 h cultivation time with 0.37 ± 0.01 g/L/h enhanced ethanol productivity (Fig. 7). This could be due to the removal of fermentative inhibitors during the detoxification process. It was observed that 16.6% of furfural, 13% of 5-HMF, 7.3% of acetic acid and 6.3% of formic acid was removed along with an average of 10% total sugar loss. Therefore, fermentation efficiency was eventually increased in detoxified hydrolyzate. These effects, which contributed to the diminution of fermentation, have been mainly attributed due to the presence of higher concentration of fermentative inhibitors (than overlimed hydrolyzate) resulting in slow down of the microbial metabolism during the non-detoxified hydrolyzate fermentation which ultimately decreases the ethanol yield.
A study conducted by Agbogbo and Wenger (2007) obtained 0.37 gp/gs ± 0.01 ethanol yield during the fermentation of different corn stover hydrolyzates which contains 1.29–1.73 g/L of furans and 6.09–7.93 g/L of acetic acid. However, the present study reports the ethanol yield of 0.40–0.42 ± 0.01 gp/gs in the presence of 0.8–0.96 g/L of furans and 2.28–2.46 g/L of acetic acid. A brief literature report on different acid pretreatment methods and their acid hydrolyzates fermentation along with ethanol yield is summarized in Table 4. As compared to the literature, the higher yield of ethanol in the present study is mainly due to low concentrations of fermentative inhibitors in the prehydrolyzates. In summary, the developed pretreatment condition significantly hydrolyzed the sorghum biomass with less carbohydrate degradation leading to low concentrations of fermentative inhibitors which ultimately increases the fermentation efficiency.
Conclusions
The optimum dilute acid pretreatment condition (121 °C, 0.2 M H2SO4 and 120 min) significantly hydrolyzed the hemicellulose in the SBMR IS11861 biomass, with 97.6% conversion efficiency, and the least decomposition of pentose sugars. The predicted values obtained through RSM based on the CCD model had shown good agreement with the experimental data. The presence of low concentration of fermentative inhibitors significantly enhanced the hydrolyzates fermentation efficiency. The ethanol yield obtained in the present study was comparatively higher than aforementioned literature reports during the fermentation of non-detoxified and detoxified hydrolyzates using Pichia stipitis NCIM 3497 wild strain.
References
Agbogbo F, Wenger K (2007) Production of ethanol from corn stover hemicellulose hydrolyzate using Pichia stipitis. J Ind Microbiol Biotechnol 34(11):723–727. doi:10.1007/s10295-007-0247-z
Barriere Y, Guillet C, Goffner D, Pichon M (2003) Genetic variation and breeding strategies for improved cell wall digestibility in annual forage crops. A review. Anim Res 52(3):193–228. doi:10.1051/animres:2003018
Brigham JS, Adney WS, Himmel ME (1996) Hemicellulases: diversity and applications. In: Wyman CE (ed) Handbook on bioethanol: production and utilization. Taylor & Francis, Washington, DC, pp 119–141
Chandel AK, Kapoor RK, Singh A, Kuhad RC (2007) Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour Technol 98(10):1947–1950. doi:10.1016/j.biortech.2006.07.047
Chen F, Dixon RA (2007) Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol 25(7):759–761. doi:10.1038/nbt1316
Dien BS, Sarath G, Pedersen JF, Sattler SE, Chen H, Funnell-Harris DL, Nichols NN, Cotta MA (2009) Improved sugar conversion and ethanol yield for forage sorghum (Sorghum bicolor L. Moench) lines with reduced lignin contents. Bioenerg res 2:153–164
Djioleu A, Carrier DJ (2016) Effects of dilute acid pretreatment parameters on sugar production during biochemical conversion of switchgrass using a full factorial design. ACS Sustain Chem Eng 4(8):4124–4130. doi:10.1021/acssuschemeng.6b00441
Ferrari MD, Neirotti E, Albornoz C, Saucedo E (1992) Ethanol production from eucalyptus wood hemicellulose hydrolysate by Pichia stipitis. Biotechnol Bioeng 40(7):753–759
Garrett E, Dvorchik B (1969) Kinetics and mechanisms of the acid degradation of the aldopentoses to furfural. J Pharm Sci 58(7):813–820
Grohmann K, Himmel M, Rivard C, Tucker M, Baker J, Torget R, Graboski M (1984) Chemical-mechanical methods for the enhanced utilization of straw. In: Sixth Symposium on Biotechnology for Fuels and Chemicals, Gatlinburg, Tennessee, USA, 15–18 May 1984, pp 137–157
Guo D, Chen F, Wheeler J, Winder J, Selman S, Peterson M, Dixon RA (2001) Improvement of in-rumen digestibility of alfalfa forage by genetic manipulation of lignin O-methyltransferases. Transgenic Res 10:457–464
Hahn-Hägerdal B, Jeppsson H, Olsson L, Mohagheghi A (1994) An interlaboratory comparison of the performance of ethanol-producing micro-organisms in a xylose-rich acid hydrolysate. Appl Microbiol Biotechnol 41(1):62–72
Hames B, Rulz R, Scarlara C, Sluiter A, Sluiter J, Templeton D (2008) Preparation of samples for compositional analysis. Laboratory Analytical Procedure (LAP), NREL/TP-510-4262
Huang C, Lin T, Guo G, Hwang W (2009) Enhanced ethanol production by fermentation of rice straw hydrolysate without detoxification using a newly adapted strain of Pichia stipitis. Bioresour Technol 100(17):3914–3920. doi:10.1016/j.biortech.2009.02.064
Jing Ping G, Bai Yan C, Guo Ming L, Hong Zhi L, Bao Zhu F, Gang S, Xiao Feng Y, Wen Xiang P (2011) Comparison of different detoxification methods for corn cob hemicelluose hydrolysate to improve ethanol production by Candida shehatae ACCC 20335. Afr J Microbiol Res 5(10):1163–1168. doi:10.5897/ajmr10.744
Jung HG, Allen MS (1995) Characteristics of plantcellwalls affectingintake and digestibility of forages by ruminants. J Anim Sci 73:2774–2790
Kamireddy SR, Li J, Abbina S, Berti M, Tucker M, Ji Y (2013) Converting forage sorghum and sunn hemp into biofuels through dilute acid pretreatment. Ind Crop Prod 49:598–609. doi:10.1016/j.indcrop.2013.06.018
Krassig H, Schurz J, Steadman RG, Schliefer K, Albrecht W, Mohring M, Schlosser H (2004) Cellulose. vol 7. Ullmann’s Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a05_375.pub2
Liu X, Ai N, Zhang H, Lu M, Ji D, Yu F, Ji J (2012) Quantification of glucose, xylose, arabinose, furfural, and HMF in corncob hydrolysate by HPLC-PDA-ELSD. Carbohydr Res 353:111–114. doi:10.1016/j.carres.2012.03.029
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686. doi:10.1016/j.biortech.2004.06.025
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev 14(2):578–597. doi:10.1016/j.rser.2009.10.003
Nigam J (2001) Ethanol production from wheat straw hemicellulose hydrolysate by Pichia stipitis. J Biotechnol 87:17–27
Nigam J (2002) Bioconversion of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to motor fuel ethanol by xylose–fermenting yeast. J Biotechnol 97:107–116
Niu M, Hou Y, Ren S, Wang W, Zheng Q, Wu W (2015) The relationship between oxidation and hydrolysis in the conversion of cellulose in NaVO3–H2SO4 aqueous solution with O2. Green Chem 17(1):335–342. doi:10.1039/c4gc00970c
Palmqvist E, Hahn-Hagerdal B (2000) Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol 74:17–24
Qi L, Mui YF, Lo SW, Lui MY, Akien GR, Horváth IT (2014) Catalytic conversion of fructose, glucose, and sucrose to 5-(hydroxymethyl)furfural and levulinic and formic acids in γ-valerolactone as a green solvent. ACS Catal 4(5):1470–1477. doi:10.1021/cs401160y
Rakesh K, Eva A, Lisbeth O (2012) Evolutionary engineering strategies to enhance tolerance of xylose utilizing recombinant yeast to inhibitors derived from spruce biomass. Biotechnol Biofuels 5(32):1–12
Rao SP, Rao SS, Seetharama N, Umakath AV, Sanjana Reddy P, Reddy BVS, Gowda CLL (2009) Sweet sorghum for biofuel and strategies for its improvement. In: Sweet sorghum for biofuel and strategies for its improvement. International Crops Research Institute for the Semi-Arid Tropics
Roberto IC, Lacis LS, Barbosa MFS, de Mancilha IM (1991) Utilization of sugar cane bagasse hemicellulose hydrolysate by Pichia stipitis for the production of ethanol. Proc Biochem 26:15–21
Rowell RM, Pettersen R, Han JS, Rowell JS, Tshabalala MA (2005) Handbook of wood chemistry and wood composites, 2nd edn. CRC Press, Boca Raton
Sanchez G, Pilcher L, Roslander C, Modig T, Galbe M, Liden G (2004) Dilute-acid hydrolysis for fermentation of the Bolivian straw material Paja Brava. Bioresour Technol 93(3):249–256. doi:10.1016/j.biortech.2003.11.003
Sluiter A, Ruiz R, Scarlata C, Sluiter J, Templeton D (2005) Determination of extractives in biomass. National Renewable Energey Laboratory, NREL/TP-510-42619
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2011) Determination of structural carbohydrates and lignin in biomass. National Renewable Energey Laboratory, NREL/TP-510-42618
Tan HT, Lee KT, Mohamed AR (2011) Pretreatment of lignocellulosic plam biomass using a solvent-ionic liquid [BMIM]Cl for glucose recovery: an optimisation study using response surface methodology. Carbohydr Polym 83:1862–1868
Torget R, Werdene P, Himmel M, Grohmann K (1990) Dilute acid pretreatment of short rotation woody and herbaceous crops. Appl Biochem Biotech 24(25):115–126
Vancov T, McIntosh S (2012) Mild acid pretreatment and enzyme saccharification of Sorghum bicolor straw. Appl Energy 92:421–428. doi:10.1016/j.apenergy.2011.11.053
Vermerris W, Saballos A, Ejeta G, Mosier NS, Ladisch MR, Carpita NC (2007) Molecular breeding to enhance ethanol production from corn and sorghum stover. Crop Sci 47:S142–S153. doi:10.2135/cropsci2007.04.0013IPBS
Vogel KP, Jung H-JG (2001) Genetic modification of herbaceous plants for feed and fuel. Crit Rev Plant Sci 20(1):15–49. doi:10.1080/20013591099173
Zhang T, Kumar R, Tsai Y-D, Elander RT, Wyman CE (2015) Xylose yields and relationship to combined severity for dilute acid post-hydrolysis of xylooligomers from hydrothermal pretreatment of corn stover. Green Chem 17(1):394–403. doi:10.1039/c4gc01283f
Zhao H, Holladay JE, Brown H, Zhang ZC (2007) Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 316:1597–1600
Acknowledgements
Authors are thankful to Mr. Gopi Kiran Mothe for his help in proof reading. Authors also would like to thank Mr. G. Radha Krishna and Dr. Sidick Basha for their valuable suggestions in carbohydrate structural representation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this research article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deshavath, N.N., Mohan, M., Veeranki, V.D. et al. Dilute acid pretreatment of sorghum biomass to maximize the hemicellulose hydrolysis with minimized levels of fermentative inhibitors for bioethanol production. 3 Biotech 7, 139 (2017). https://doi.org/10.1007/s13205-017-0752-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0752-3