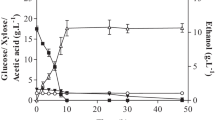
Abstract
Acetone–butanol–ethanol (ABE) was produced in Clostridium saccharoperbutylacetonicum N1-4 using sago starch as a substrate, and the effects of nutrient supplementation on ABE production were studied. Using sago starch as a substrate led to higher butanol production in C. saccharoperbutylacetonicum N1-4 when compared to other starchy materials as substrates. TYA medium supplemented with sago starch led to enhanced ABE and butanol production; however, P2 medium improved ABE production more than TYA medium. The highest ABE and butanol production was achieved with P2 medium supplemented with 70 g/L of sago starch. Using these fermentation conditions, ABE and butanol were produced at 16.65 and 9.83 g/L, respectively, and productivity was 0.15 g/L h for ABE and 0.09 g/L h for butanol. Using P2 medium supplemented with either 50 g/L enzymatically hydrolyzed sago starch or 70 g/L sago starch without hydrolysis, 9.81 and 9.83 g/L butanol were produced, respectively. These results reveal the importance of media formulation in the biobutanol fermentation of sago starch by C. saccharoperbutylacetonicum N1-4.






Similar content being viewed by others
References
Ahmad FB, Williams PA, Doublier JL, Durand S, Buleon A (1999) Physico-chemical characterisation of sago starch. Carbohydrate Polymers 38(4):361–370
Al-Shorgani N, Ali E, Kalil M, Yusoff W (2011) Bioconversion of butyric acid to butanol by Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) in a limited nutrient medium. BioEnergy Res. doi:10.1007/s12155-011-9126-6
Annous BA, Blaschek HP (1990) Regulation and localization of amylolytic enzymes in Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 56(8):2559–2561
Cone JW, Wolters MGE (1990) Some properties and degradability of isolated starch granules. Starch-Stärke 42(8):298–301
Dürre P (2007) Biobutanol: an attractive biofuel. Biotechnol J 2(12):1525–1534
Ennis BM, Maddox IS (1985) Use of Clostridium acetobutylicum P262 for production of solvents from whey permeate. Biotechnol Lett 7(8):601–606
Ezeji T, Groberg M, Qureshi N, Blaschek H (2003) Continuous production of butanol from starch-based packing peanuts. Appl Biochem Biotechnol 106(1):375–382
Formanek J, Mackie R, Blaschek HP (1997) Enhanced butanol production by Clostridium beijerinckii BA101 grown in semidefined P2 medium containing 6 percent maltodextrin or glucose. Appl Environ Microbiol 63:2306–2310
Harriman N (1998) Promoting the conservation and use of underutilized and neglected crops. 13. Sago palm, Metroxylon sagu. Econ Bot 52(1):77–77
Hipolito CN, Crabbe E, Badillo CM, Zarrabal OC, Morales MA, Flores GP, Hernández CMA, Ishizaki A (2008) Bioconversion of industrial wastewater from palm oil processing to butanol by Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564). J Cleaner Prod 16(5):632–638
Ishizaki A (1997) Solution of environmental problems through biomass conversion using microbial technology. In: Fuels and Chemicals from Biomass American Chemical Society, pp 336–344
Ishizaki A, Michiwaki S, Crabbe E, Kobayashi G, Sonomoto K, Yoshino S (1999) Extractive acetone-butanol-ethanol fermentation using methylated crude palm oil as extractant in batch culture of Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564). J Biosci Bioeng 87:352–356
Jones DT, Woods DR (1986) Acetone-butanol fermentation revisited. Microbiol Rev 50(4):484–524
Kitahata S, Okada S (1974) Action of cyclodextrin glycosyltransferase from Bacillus megaterium strain No. 5 on starch. Agric Biol Chem 38:2413–2417
Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS (2008) Fermentative butanol production by clostridia. Biotechnol Bioeng 101(2):209–228
Liew ST, Arbakariya A, Rosfarizan M, Raha AR (2006) Production of solvent (acetone-butanol-ethanol) in continuous fermentation by Clostridium saccharobutylicum DSM 13864 using gelatinised sago starch as a carbon source. Malays J Microbiol 2(2):42–50
Ma Y, Cai C, Wang J, Sun DW (2006) Enzymatic hydrolysis of corn starch for producing fat mimetics. J Food Eng 73(3):297–303
Madihah M, Ariff A, Khalil M, Suraini A, Karim M (2001) Anaerobic fermentation of gelatinized sago starch-derived sugars to acetone—1-butanol—ethanol solvent by Clostridium acetobutylicum. Folia Microbiol 46(3):197–204
Mitchell WJ (1997) Physiology of carbohydrate to solvent conversion by clostridia. In: Poole RK (ed) Advances in microbial physiologyAcademic, New York, pp 31–130
Nolasco HC, Crabbe EKG, Sonomoto K, Ishizaki A (2000) pH-dependent continuous lactic acid fermentation by Lactococcus lactis IO-1 using hydrolyzed sago starch. J Fac Agric Kyushu Univ 44(3–4):367–375
Paquet V, Croux C, Goma G, Soucaille P (1991) Purification and characterization of the extracellular alpha-amylase from Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 57(1):212–218
Qureshi N, Blaschek HP (2001) Recovery of butanol from fermentationbroth by gas stripping. Renew Energy 22:557–564
Rosfarizan M, Madihah S, Ariff AB (1998) Isolation of a kojic acid-producing fungus capable of using starch as a carbon source. Lett Appl Microbiol 26(1):27–30
Shariffa YN, Karim AA, Fazilah A, Zaidul ISM (2009) Enzymatic hydrolysis of granular native and mildly heat-treated tapioca and sweet potato starches at sub-gelatinization temperature. Food Hydrocolloids 23(2):434–440
Shen CR, Lan EI, Dekishima Y, Baez A, Cho KM, Liao JC (2011) Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli. Appl Environ Microbiol 77(9):2905–2915
Soni BK, Soucaille P, Goma G (1987)Continuous acetone-butanol fermentation: influence of vitamins on the metabolic activity of Clostridium acetobutylicumAppl Microbiol Biotechnol 27:1–5
Suraini AA (2002) Sago starch and its utilisation. Soc Biotechnol Jpn 94(6):526–529
Swinkels JJM (1985) Sources of starch, its chemistry and physics. In: Beynum GMAV, Roels JA (eds) Starch conversion technology. Marcel Dekker, New York, pp 15–45
Syed QA, Nadeem M, Nelofer R (2008) Enhanced butanol production by mutant dtrains of Clostridium acetobutylicum in molasses medium. Turk J Biochem 33(1):25–30
Thang V, Kanda K, Kobayashi G (2010) Production of acetone–butanol–ethanol (ABE) in direct fermentation of cassava by Clostridium saccharoperbutylacetonicum N1-4. Appl Biochem Biotechnol 161(1):157–170
Wang WJ, Powell AD, Oates CG (1996) Sago starch as a biomass source: raw sago starch hydrolysis by commercial enzymes. Bioresour Technol 55(1):55–61
Welsh FW, Williams RE, Veliky IA (1986) A note on the effect of nitrogen source on growth of and solvent production by Clostridium acetobutylicum. J Appl Microbiol 61(5):413–419
Yuan Y, Zhang L, Dai Y, Yu J (2007) Physicochemical properties of starch obtained from Dioscorea nipponica Makino comparison with other tuber starches. J Food Eng 82(4):436–442
Acknowledgments
We would like to thank Prof. Dr Yoshino Sadazo, Kyushu University, Japan, who provided us with C. saccharoperbutylacetonicum N1-4. This research was supported by the UKM-GUP-KPB-08-32-128 grant.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 584 kb)
Rights and permissions
About this article
Cite this article
Al-Shorgani, N.K.N., Kalil, M.S. & Yusoff, W.M.W. Fermentation of sago starch to biobutanol in a batch culture using Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564). Ann Microbiol 62, 1059–1070 (2012). https://doi.org/10.1007/s13213-011-0347-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0347-x