Abstract
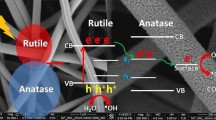
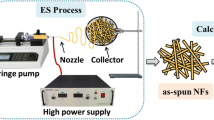
In this study, a new hierarchical nanostructure consisting of zinc oxide (ZnO) and titanium dioxide (TiO2) was prepared by an electrospinning process followed by a hydrothermal technique for use as a photocatalyst for dye degradation. First, the electrospinning of a colloidal solution consisting of titanium isopropoxide/poly(vinyl acetate)/zinc nanoparticles was performed to produce polymeric nanofibers embedded in solid nanoparticles. Calcination of the obtained electrospun nanofiber mats in air at 600 °C produced TiO2 nanofibers containing ZnO nanoparticles (i.e., ZnO-doped TiO2 nanofibers). The ZnO nanoparticles formed were then exploited as seeds to produce the outgrowth ZnO branches around the TiO2 nanofibers using the hydrothermal technique. Photodegradation of methyl red and rhodamine B (RB) dyes was examined individually using four photocatalysts: ZnO nanoparticles prepared by the same hydrothermal technique, pristine TiO2 nanofibers, ZnO-doped TiO2 nanofibers and the produced nanostructure. The results showed that the introduced ZnO-TiO2 hierarchical nanostructure can eliminate all the methyl red dye within 90 min and the rhodamine B dye within 105 min. However, the other three nanostructures could not totally remove any of the dyes, even after 3 h. Therefore, the introduced nanostructure has higher photocatalytic activity than any of its ingredients individually, which highlights the advantages of synthesizing this novel structure.
Similar content being viewed by others
References
J. P. Jadhav, G. K. Parshetti, S. D. Kalme, and S. P. Govindwar, Chemosphere, 68, 394 (2007).
F. Han, V. S. R. Kambala, M. Srinivasan, D. Rajarathnam, and R. Naidu, Appl. Catal. A: Gen., 359, 25 (2009).
M. A. Rauf and S. S. Ashraf, Chem. Eng. J., 151, 10 (2009).
X. Li, K. Lv, K. Deng, J. Tang, R. Su, J. Sun, and L. Chen, Mater. Sci. Eng. B, 158, 40 (2009).
A. L. Linsebigler, G. Lu, and J. T. Yates, Chem. Rev., 95, 735 (1995).
V. Sukharev and R. Kershaw, J. Photochem. Photobiol. A, 98, 165 (1996).
N. Serpone, P. Maruthamuthu, P. Pichat, E. Pelizzetti, and H. Hidaka, J. Photochem. Photobiol. A, 85, 247 (1995).
X. Fu, L. A. Clark, Q. Yang, and M. A. Anderson, Environ. Sci. Technol., 30, 647 (1996).
Y. Shaogui, Q. Xie, L. Xinyong, L. Yazi, C. Shuo, and C. Guohua, Phys. Chem. Chem. Phys., 6, 659 (2004).
N. Sobana and M. Swaminathan, Sep. Purif. Technol., 56, 101 (2007).
C. Shifu, Z. Wei, Z. Sujuan, and L. Wei, Chem. Eng. J., 148, 263 (2009).
Y. J. Yang, J. G. Zhao, and S. Hu, Electrochem. Commun., 9, 2681 (2007).
H. Y. Yap, B. Ramaker, A. V. Sumant, and R. W. Carpick, Diamond Relat. Mater., 15, 1622 (2006).
P. M. Ajayan, O. Stephan, P. Redlich, and C. Colliex, Nature, 375, 769 (1995).
M. Knez et al., Nano Lett., 3, 1079 (2003).
N. V. Quy, N. D. Hoa, W. J. Yu, Y. S. Cho, G. S. Choi, and D. J. Kim, Nanotechnology, 17, 2156 (2006).
X. M. Yang, T. Y. Dai, Z. X. Zhu, and Y. Lu, Polymer, 48, 4021 (2007).
H. Hosseinkhania, M. Hosseinkhani, F. Tian, H. Kobayashi, and Y. Tabata, Biomaterials, 27, 4079 (2006).
J. D. Hartgerink, E. Beniash, and S. I. Stupp, Science, 294, 1684 (2001).
N. R. Chiou, C. Lu, J. J. Guan, L. J. Lee, and A. J. Epstein, Nature Nanotechnology, 147, 354 (2007).
R. Haggenmueller, F. Du, J. E. Fischer, and K. I. Winey, Polymer, 47, 2381 (2006).
X. Y. Zhang, W. J. Goux, and S. K. Manohar, J. Am. Chem. Soc., 126, 4502 (2004).
C. H. Kim, Y. H. Jung, H. Y. Kim, D. R. Lee, N. Dharmaraj, and K. E. Choi, Macromol. Res., 14, 59 (2006).
Y. H. Jung, H. Y. Kim, D. R. Lee, S. Y. Park, and M. S. Khil, Macromol. Res., 13, 385 (2005).
F. A. Sheikh, N. A. M. Barakat, M. A. Kanjwal, D. K. Park, S. J. Park, and H. Y. Kim, Macromol. Res., 18, 59 (2010).
F. A. Sheikh, N. A. M. Barakat, M. A. Kanjwal, A. A. Chaudhari, I. -H. Jung, J. H. Lee, and H. Y. Kim, Macromol. Res., 17, 688 (2009).
W. Sigmund, J. Yuh, H. Park, V. Maneeratana, G. Pyrgiotakis, A. Daga, J. Taylor, and J. C. Nino, J. Am. Ceram. Soc., 89, 395 (2006).
J. Lee, B. S. Kang, B. Hicks, T. F. Chancellor, B. H. Chu, H. T. Wang, B. G. Keselowsky, F. Ren, and T. P. Lele, Biomaterials, 29, 3743 (2008).
M. A. Kanjwal, N. A. M. Barakat, F. A. Sheikh, M. S. Khil, and H. Y. Kim, Int. J. Appl. Ceram. Technol., 1 (2009).
L. E. Greene, M. Law, J. Goldberger, F. Kim, J. C. Johnson, and Y. Zhang, Angew. Chem. Int. Ed. Engl., 42, 3031 (2003).
B. S. Kang, S. J. Pearton, and F. Ren, Appl. Phys. Lett., 90, 084104 (2007).
C. Pacholski, A. Kornowski, and H. Weller, Angew. Chem. Int. Ed. Engl., 41, 1188 (2002).
D. L. Liao, C. A. Badour, and B. Q. Liao, J. Photochem. Photobiol. A, 194, 11 (2008).
D. Robert, Catal. Today, 122, 20 (2007).
M. W. Xiao, L. S. Wang, Y. D. Wu, X. J. Huang, and Z. Dang, Nanotechnology, 19, 015706 (2008).
J. C. Xu, M. Lu, X. Y. Guo, and H. L. Li, J. Mol. Catal., 226, 123 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanjwal, M.A., Barakat, N.A.M., Sheikh, F.A. et al. Photocatalytic activity of ZnO-TiO2 hierarchical nanostructure prepared by combined electrospinning and hydrothermal techniques. Macromol. Res. 18, 233–240 (2010). https://doi.org/10.1007/s13233-010-0303-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-010-0303-9