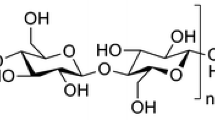
Abstract
Chitin the second most abundant polysaccharide is synthesized by an enormous number of living organisms including fungi and insects. These biopolymers have found many applications in different areas such as: packaging material, membrane for removal of metal ions, dyes and pigments in waste water engineering; anti-cholesterol, fat binding, preservative and food additive in food industry; seed and fertilizer coating, controlled agrochemical release in agriculture; surface treatment, photographic paper in pulp and paper industry; moisturizer, body creams and lotions in cosmetics and toiletries. It has also found wide applications in biomedical such as tissue engineering, drug delivery, wound dressing, scaffolds, cancer diagnosis, etc. The majority of these versatile applications are coming of its non-toxicity, biocompatibility and biodegradability. Chitin is also easily processed as gel, membrane, and nanofiber. This review emphasizes an extensive bibliography of recent basic and applied research and investigations on the aspects of this interesting biopolymer including the recovery, preparation, modification and application of chitin and its derivatives and related compounds. A new class of biocompatible and biodegradable chitin-based polyurethane (PU) elastomer was also introduced and reviewed in this study and it was found that by incorporation of chitin into the PU elastomer backbone, biocompatibility and degradation rate of the final elastomer improved. PUs are one of the synthetic biocompatible polymers with excellent physical and mechanical properties. Combination of this polymer with chitin resulted to a new tailor-made biocompatible and biodegradable polymer with improved properties. These polymers have potential applications in various applications including biomedical.
















Similar content being viewed by others
References
Li H, Greene LH (2010) Sequence and structural analysis of the chitinase insertion domain reveals two conserved motifs involved in chitin-binding. PLoS One 5:1–11
Arbia W, Arbia L, Adour L, Amrane A (2013) Chitin extraction from crustacean shells using biological methods—a review. Food Technol Biotechnol 51:12–25
Ravi Kumar MNV (2000) A review of chitin and chitosan applications. React Funct Polym 46:1–27
Krajewska B (2004) Application of chitin- and chitosan-based materials for enzyme immobilizations: a review. Enzym Microbial Technol 35:126–139
Zhao Y, Kim Y, Oh K, Nguyen N, Park R (2010) Production and characterization of extracellular chitin deacetylase from Absidia corymbifera DY-9. J Korean Soc Appl Biol Chem 53:119–126
Honarkar H, Barikani M (2009) Applications of biopolymers. I: chitosan. Monatsh Chem 140:1403–1420
Ehrlich H, Steck E, Ilan M, Maldonado M, Muricy G, Bavestrello G, Kljajic Z, Carballo JL, Schiaparelli S, Ereskovsky A, Schupp P, Born R, Worch H, Bazhenov VV, Kurek D, Varlamov V, Vyalikh D, Kummer K, Sivkov VV, Molodtsov SL, Meissner H, Richter G, Hunoldt S, Kammer M, Paasch S, Krasokhin V, Patzke G, Brunner E, Richter W (2010) Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part II: biomimetic potential and applications. Int J Biolog Macromol 47:141–145
Camci-Unal G, Pohl NLB (2009) Quantitative determination of heavy metal contaminant complexation by the carbohydrate polymer chitin. J Chem Eng Data 55:1117–1121
Muzzarelli RAA, Boudrant J, Meyer D, Manno N, DeMarchis M, Paoletti MG (2012) Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: a tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr Polym 87:995–1012
Jayakumar R, Menon D, Manzoor K, Nair SV, Tamura H (2010) Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydr Polym 82:227–232
Merzendorfer H (2011) The cellular basis of chitin synthesis in fungi and insects: common principles and differences. Eur J Cell Biol 90:759–769
Nogi M, Kurosaki F, Yano H, Takano M (2010) Preparation of nanofibrillar carbon from chitin nanofibers. Carbohydr Polym 81:919–924
Gortari MC, Hours RA (2013) Biotechnological processes for chitin recovery out of crustacean waste: a mini-review. Electron J Biotechnol 16:1–14
Mao X, Zhang J, Kan F, Gao Y, Lan J, Zhang X, Hu Z, Li Y, Lin H (2013) Antioxidant production and chitin recovery from shrimp head fermentation with Streptococcus thermophilus. Food Sci Biotechnol 22:1023–1032
Bedekar AN, Pise AC, Thatte CS, Rathnam MV (2010) Study on optimization of carboxymethylation of chitosan obtained from Squilla chitin. Asian J Chem 22:7675–7682
Pillai CKS, Paul W, Chandra P, Sharma CP (2009) Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci 34:641–678
Yaghobi N, Mirzadeh H (2004) Enhancement of chitin_s degree of deacetylation by multistage alkali treatments. Iran Polym J 13:131–136
Valdez-Peña A, Espinoza-Perez J, Sandoval-Fabian G, Balagurusamy N, Hernandez-Rivera A, De-la-Garza-Rodriguez I, Contreras-Esquivel J (2010) Screening of industrial enzymes for deproteinization of shrimp head for chitin recovery. Food Sci Biotechnol 19:553–557
Gartner C, Peláez CA, López BL (2013) Characterization of chitin and chitosan extracted from shrimp shells by two methods. e-Polymers 10:748–763
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Nitschke J, Altenbach H-J, Malolepszy T, Mölleken H (2011) A new method for the quantification of chitin and chitosan in edible mushrooms. Carbohydr Res 346:1307–1310
Ghorbel-Bellaaj O, Hmidet N, Jellouli K, Younes I, Maâlej H, Hachicha R, Nasri M (2011) Shrimp waste fermentation with Pseudomonas aeruginosa A2: optimization of chitin extraction conditions through Plackett-Burman and response surface methodology approaches. Int J Biolog Macromol 48:596–602
Junianto J, Wahyuntari B, Setyahadi S (2013) Selection of method for microbiological extraction of chitin from shrimp shells. Microbiology 7:75–83
Lavall RL, Assis OBG, Campana-Filho SP (2007) β-Chitin from the pens of Loligo sp.: extraction and characterization. Bioresour Technol 98:2465–2472
Di Mario F, Rapanà P, Tomati U, Galli E (2008) Chitin and chitosan from Basidiomycetes. Int J Biolog Macromol 43:8–12
Yen MT, Mau JL (2007) Selected physical properties of chitin prepared from shiitake stipes. LWT Food Sci Technol 40:558–563
Chang PR, Jian R, Yu J, Ma X (2010) Starch-based composites reinforced with novel chitin nanoparticles. Carbohydr Polym 80:420–425
Ifuku S, Nogi M, Abe K, Yoshioka M, Morimoto M, Saimoto H, Yano H (2011) Simple preparation method of chitin nanofibers with a uniform width of 10–20 nm from prawn shell under neutral conditions. Carbohydr Polym 84:762–764
Maghami GG, Roberts GAF (1988) Evaluation of the viscometric constants for chitosan. Makromol Chem 189:195–200
Jayakumar R, Prabaharan M, Nair SV, Tokura S, Tamura H, Selvamurugan N (2010) Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog Mater Sci 55:675–709
Zohuriaan-Mehr MJ (2005) Advances in chitin and chitosan modification through graft copolymerization: a comprehensive review. Iran Polym J 14:235–265
Wang J, Wang Z, Li J, Wang B, Liu J, Chen P, Miao M, Gu Q (2012) Chitin nanocrystals grafted with poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and their effects on thermal behavior of PHBV. Carbohydr Polym 87:784–789
Hattori T, Sakabe Y, Ogata M, Michishita K, Dohra H, Kawagishi H, Totani K, Nikaido M, Nakamura T, Koshino H, Usui T (2012) Enzymatic synthesis of an α-chitin-like substance via lysozyme-mediated transglycosylation. Carbohydr Res 347:16–22
Uddin AJ, Fujie M, Sembo S, Gotoh Y (2012) Outstanding reinforcing effect of highly oriented chitin whiskers in PVA nanocomposites. Carbohydr Polym 87:799–805
Philippova OE, Korchagina EV, Volkov EV, Smirnov VA, Khokhlov AR, Rinaudo M (2012) Aggregation of some water-soluble derivatives of chitin in aqueous solutions: role of the degree of acetylation and effect of hydrogen bond breaker. Carbohydr Polym 87:687–694
Wu J, Liang S, Dai H, Zhang X, Yu X, Cai Y, Zhang L, Wen N, Jiang B, Xu J (2010) Structure and properties of cellulose/chitin blended hydrogel membranes fabricated via a solution pre-gelation technique. Carbohydr Polym 79:677–684
Hu X, Tang Y, Wang Q, Li Y, Yang J, Du Y, Kennedy JF (2011) Rheological behaviour of chitin in NaOH/urea aqueous solution. Carbohydr Polym 83:1128–1133
Jaworska MM (2011) Chitin deacetylase product inhibition. Biotechnol J 6:244–247
Pareek N, Vivekanand V, Dwivedi P, Singh RP (2011) Penicillium oxalicum SAEM-51: a mutagenised strain for enhanced production of chitin deacetylase for bioconversion to chitosan. New Biotechnol 28:118–124
Cai J, Yang J, Du Y, Fan L, Qiu Y, Li J, Kennedy JF (2006) Purification and characterization of chitin deacetylase from Scopulariopsis brevicaulis. Carbohydr Polym 65:211–217
Pareek N, Vivekanand V, Saroj S, Sharma AK, Singh RP (2012) Purification and characterization of chitin deacetylase from Penicillium oxalicum SAEM-51. Carbohydr Polym 87:1091–1097
Wang S, Ye X, Chen J, Rao P (2012) A novel chitinase isolated from Vicia faba and its antifungal activity. Food Res Int 45:116–122
Narayanan K, Chopade N, Raj PV, Subrahmanyam VM, Rao JV (2013) Fungal chitinase production and its application in biowaste management. J Sci Indust Res (JSIR) 72:393–399
Suginta W (2007) Identification of chitin binding proteins and characterization of two chitinase isoforms from Vibrio alginolyticus 283. Enzyme Microbial Technol 41:212–220
Sureshkumar M, Lee CK (2011) Polydopamine coated magnetic-chitin (MCT) particles as a new matrix for enzyme immobilization. Carbohydr Polym 84:775–780
Kopparapu NK, Liu Z, Yan Q, Jiang Z, Zhang S (2011) A novel thermostable chitinase (PJC) from pomegranate (Punica granatum) juice. Food Chem 127:1569–1575
Ilankovan P, Hein S, Ng CH, Trung TS, Stevens WF (2006) Production of N-acetyl chitobiose from various chitin substrates using commercial enzymes. Carbohydr Polym 63:245–250
Barikani M, Zia KM, Bhatti IA, Zuber M, Bhatti HN (2008) Molecular engineering and properties of chitin based shape memory polyurethanes. Carbohydr Polym 4:621–626
Zia KM, Bhatti IA, Barikani M, Zuber M, Sheikh MA (2008) XRD studies of chitin-based polyurethane elastomers. Int J Biolog Macromol 43:136–141
Zia KM, Barikani M, Bhatti IA, Zuber M, Bhatti HN (2008) Synthesis and characterization of novel biodegradable thermally stable chitin based polyurethane elastomers. J Appl Polym Sci 110:769–776
Zia KM, Zuber M, Bhatti IA, Barikani M, Sheikh MA (2009) Evaluation of biocompatibility and mechanical behaviour of polyurethane elastomers based on chitin/1,4-butane diol blends. Int J Biolog Macromol 44:18–22
Zia KM, Zuber M, Bhatti IA, Britani M, Sheikh MA (2009) Evaluation of biocompatibility and mechanical behavior of chitin based polyurethane elastomers. Part II: effect of diisocyanate structure. Int J Biolog Macromol 44:23–28
Zia KM, Barikani M, Zuber M, Bhatti IA, Barmar M (2009) Surface characteristics of polyurethane elastomers based on chitin/1,4-butanediol blends. Int J Biolog Macromol 44:182–185
Zia KM, Barikani M, Zuber M, Bhatti IA, Barmar M (2009) XRD studies of UV irradiated chitin-based polyurethane elastomers. Carbohydr Polym 77:54–58
Zia KM, Barikani M, Khalid AM, Honarkar H, ul-Haq E (2009) Surface characteristics of UV-irradiated chitin-based polyurethane elastomers. Carbohydr Polym 77:621–627
Barikani M, Honarkar H, Barikani M (2009) Synthesis and characterization of polyurethane elastomers based on chitosan and poly(ε-caprolactone). J Appl Polym Sci 112:3157–3165
Barikani M, Honarkar H, Barikani M (2010) Synthesis and characterization of chitosan-based polyurethane elastomer dispersions. Monatsh Chem 141:653–659
Zia KM, Zuber M, Barikani M, Hussain R, Jamil T, Anjum S (2011) Cytotoxicity and mechanical behavior of chitin–bentonite clay based polyurethane bio-nanocomposites. Int J Biolog Macromol 49:1131–1136
Zia KM, Zuber M, Saif MJ, Jawaid M, Kashif M, Shahid M, Anjum MN, Ahmad MN (2013) Chitin based polyurethanes using hydroxyl terminated polybutadiene. Part III: surface characteristics. Int J Biolog Macromol 62:670–676
Zia KM, Zuber M, Barikani M, Bhatti IA, Khan MB (2009) Surface characteristics of chitin-based shape memory polyurethanes elastomers. Colloid Surf B Biointerfaces 72:248–252
Zia KM, Zuber M, Barikani M, Jabbar A, Khosa MK (2010) XRD pattern of chitin based polyurethane bio-nanocomposites. Carbohydr Polym 80:539–543
Zia KM, Zuber M, Mahboob S, Sultana T, Sultana S (2010) Surface characteristics of UV-irradiated chitin-based shape memory polyurethanes. Carbohydr Polym 80:229–234
Saralegi A, Fernandes SCM, Alonso-Varona A, Palomares T, Foster EJ, Weder C, Eceiza A, Corcuera MA (2013) Shape-memory bionanocomposites based on chitin nanocrystals and thermoplastic polyurethane with a highly crystalline soft segment. Biomacromolecules 14:4475–4482
Tsioptsias C, Tsivintzelis I, Papadopoulou L, Panayiotou C (2009) A novel method for producing tissue engineering scaffolds from chitin, chitin-hydroxyapatite, and cellulose. Mater Sci Eng C 29:159–164
Sashiwa H, Aiba S-I (2004) Chemically modified chitin and chitosan as biomaterials. Prog Polym Sci 29:887–908
Kousalya GN, Gandhi MR, Meenakshi S (2010) Preparation of modified chitin for the removal of chromium (VI). Bioremediation J 14:208–218
Matsui M, Ono L, Akcelrud L (2012) Chitin/polyurethane networks and blends: evaluation of biological application. Polym Test 31:191–196
Shahidi F, Arachchi JKV, Jeon YJ (1999) Food applications of chitin and chitosans. Trends Food Sci Technol 10:37–51
Dai DH, Li W, Hu WL, Sa XY (2011) Effect of medium composition on the synthesis of chitinase and chitin deacetylase from thermophilic Paenibacillus sp. Hul. Proc Environ Sci 8:620–628
Dolphen R, Thiravetyan P (2011) Adsorption of melanoidins by chitin nanofibers. Chem Eng J 166:890–895
Li X, Li X, Ke B, Shi X, Du Y (2011) Cooperative performance of chitin whisker and rectorite fillers on chitosan films. Carbohydr Polym 85:747–752
Harikrishnan R, Kim JS, Balasundaram C, Heo MS (2012) Immunomodulatory effects of chitin and chitosan enriched diets in Epinephelus bruneus against Vibrio alginolyticus infection. Aquaculture 326–329:46–52
Askarian F, Zhou Z, Olsen RE, Sperstad S, Ringø E (2012) Culturable autochthonous gut bacteria in Atlantic salmon (Salmo salar L.) fed diets with or without chitin. Characterization by 16S rRNA gene sequencing, ability to produce enzymes and in vitro growth inhibition of four fish pathogens. Aquaculture 326–329:1–8
Ding F, Shi X, Li X, Cai J, Duan B, Du Y (2012) Homogeneous synthesis and characterization of quaternized chitin in NaOH/urea aqueous solution. Carbohydr Polym 87:422–426
Kong CS, Kim JA, Bak SS, Byun HG, Kim SK (2011) Anti-obesity effect of carboxymethyl chitin by AMPK and aquaporin-7 pathways in 3T3-L1 adipocytes. J Nutrition Biochem 22:276–281
Neyrinck AM, Possemiers S, Verstraete W, De Backer F, Cani PD, Delzenne NM (2012) Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutrition Biochem 23:51–59
Sánchez R, Stringari GB, Franco JM, Valencia C, Gallegos C (2011) Use of chitin, chitosan and acylated derivatives as thickener agents of vegetable oils for bio-lubricant applications. Carbohydr Polym 85:705–714
Tzoumaki MV, Moschakis T, Biliaderis CG (2011) Mixed aqueous chitin nanocrystal—whey protein dispersions: microstructure and rheological behaviour. Food Hydrocoll 25:935–942
Sharp RG (2013) A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 3:757–793
Dutta PK, Dutta J, Tripathi VS (2004) Chitin and chitosan: chemistry, properties and applications. J Sci Indust Res 63:20–31
Wang SL, Liang TW, Yen YH (2011) Bioconversion of chitin-containing wastes for the production of enzymes and bioactive materials. Carbohydr Polym 84:732–742
Yang MH, Kuo CH, Hsieh WC, Ku KL (2010) Investigation of microbial elicitation of trans-resveratrol and trans-piceatannol in peanut callus led to the application of chitin as a potential elicitor. J Agricul Food Chem 58:9537–9541
Shimizu T, Nakano T, Takamizawa D, Desaki Y, Ishii-Minami N, Nishizawa Y, Minami E, Okada K, Yamane H, Kaku H, Shibuya N (2010) Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J 64:204–214
Jayakumar R, Prabaharan M, Nair SV, Tamura H (2010) Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv 28:142–150
Peter M, Sudheesh Kumar PT, Binulal NS, Nair SV, Tamura H, Jayakumar R (2009) Development of novel α-chitin/nanobioactive glass ceramic composite scaffolds for tissue engineering applications. Carbohydr Polym 78:926–931
Nagahama H, Kashiki T, Nwe N, Jayakumar R, Furuike T, Tamura H (2008) Preparation of biodegradable chitin/gelatin membranes with GlcNAc for tissue engineering applications. Carbohydr Polym 73:456–463
Nagahama H, Nwe N, Jayakumar R, Koiwa S, Furuike T, Tamura H (2008) Novel biodegradable chitin membranes for tissue engineering applications. Carbohydr Polym 73:295–302
Sudheesh Kumar PT, Srinivasan S, Lakshmanan V-K, Tamura H, Nair SV, Jayakumar R (2011) β-Chitin hydrogel/nano hydroxyapatite composite scaffolds for tissue engineering applications. Carbohydr Polym 85:584–591
Jayakumar R, Ramachandran R, Sudheesh Kumar PT, Divyarani VV, Srinivasan S, Chennazhi KP, Tamura H, Nair SV (2011) Fabrication of chitin–chitosan/nano ZrO2 composite scaffolds for tissue engineering applications. Int J Biolog Macromol 49:274–280
Madhumathi K, Sudheesh Kumar PT, Kavya KC, Furuike T, Tamura H, Nair SV, Jayakumar R (2009) Novel chitin/nanosilica composite scaffolds for bone tissue engineering applications. Int J Biolog Macromol 45:289–292
Tamura H, Furuike T, Nair SV, Jayakumar R (2011) Biomedical applications of chitin hydrogel membranes and scaffolds. Carbohydr Polym 84:820–824
Zhao J, Duan K, Zhang JW, Guo LY, Weng J (2011) Preparation of highly interconnected porous hydroxyapatite scaffolds by chitin gel-casting. Mater Sci Eng C 31:697–701
Jayakumar R, Ramachandran R, Divyarani VV, Chennazhi KP, Tamura H, Nair SV (2011) Fabrication of chitin–chitosan/nano TiO2-composite scaffolds for tissue engineering applications. Int J Biolog Macromol 48:336–344
Dufresne A, Thomas S, Pothan LA (2013) Biopolymer nanocomposites: processing, properties, and applications. Wiley Series on Polymer Engineering and Technology (Book 8) 1st edn, Wiley, New York
Freier T, Montenegro R, Shan Koh H, Shoichet MS (2005) Chitin-based tubes for tissue engineering in the nervous system. Biomaterials 26:4624–4632
Ge Z, Baguenard S, Lim LY, Wee A, Khor E (2004) Hydroxyapatite—chitin materials as potential tissue engineered bone substitutes. Biomaterials 25:1049–1058
Kumar PTS, Srinivasan S, Lakshmanan VK, Tamura H, Nair SV, Jayakumar R (2011) Synthesis, characterization and cytocompatibility studies of α-chitin hydrogel/nano hydroxyapatite composite scaffolds. Int J Biolog Macromol 49:20–31
Ji YL, Wolfe PS, Rodriguez IA, Bowlin GL (2012) Preparation of chitin nanofibril/polycaprolactone nanocomposite from a nonaqueous medium suspension. Carbohydr Polym 87:2313–2319
Silva SS, Duarte ARC, Carvalho AP, Mano JF, Reis RL (2011) Green processing of porous chitin structures for biomedical applications combining ionic liquids and supercritical fluid technology. Acta Biomater 7:1166–1172
Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H (2011) Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv 29:322–337
Muzzarelli RAA (2009) Chitin and chitosan for the repair of wounded skin, nerve, cartilage and bone. Carbohydr Polym 76:167–182
Kumar PTS, Abhilash S, Manzoor K, Nair SV, Tamura H, Jayakumar R (2010) Preparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applications. Carbohydr Polym 80:761–767
Min SK, Lee SC, Hong SD, Chung CP, Park WH, Min BM (2010) The effect of a laminin-5-derived peptide coated onto chitin microfibers on re-epithelialization in early-stage wound healing. Biomaterials 31:4725–4730
Azuma K, Osaki T, Wakuda T, Ifuku S, Saimoto H, Tsuka T, Imagawa T, Okamoto Y, Minami S (2012) Beneficial and preventive effect of chitin nanofibrils in a dextran sulfate sodium-induced acute ulcerative colitis model. Carbohydr Polym 87:1399–1403
Gao X, Wang R, Liang J, Xiang J, Huang J, Wei M, Zeng X (2012) Inhibition of P-selectin-mediated inflammation cell adhesion by 6SCM-chitin. J Taiwan Inst Chem Eng 43:24–28
Noh HK, Lee SW, Kim JM, Oh JE, Kim KH, Chung CP, Choi SC, Park WH, Min BM (2006) Electrospinning of chitin nanofibers: degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials 27:3934–3944
García Mir V, Heinämäki J, Antikainen O, Iraizoz Colarte A, Airaksinen S, Karjalainen M, Bilbao Revoredo O, Nieto OM, Yliruusi J (2011) Effects of moisture on tablet compression of chitin. Carbohydr Polym 86:477–483
Rejinold NS, Nair A, Sabitha M, Chennazhi KP, Tamura H, Nair SV, Jayakumar R (2012) Synthesis, characterization and in vitro cytocompatibility studies of chitin nanogels for biomedical applications. Carbohydr Polym 87:943–949
Huang X, Wu Y, Wei S, Chen Q, Liu C (2012) The effect of carboxymethyl chitin on sustained drug release of aspirin tablet. Mater Lett 66:206–208
Dev A, Mohan JC, Sreeja V, Tamura H, Patzke GR, Hussain F, Weyeneth S, Nair SV, Jayakumar R (2010) Novel carboxymethyl chitin nanoparticles for cancer drug delivery applications. Carbohydr Polym 79:1073–1079
Manzoor K, Johny S, Thomas D, Setua S, Menon D, Nair S (2009) Bio-conjugated luminescent quantum dots of doped ZnS: a cyto-friendly system for targeted cancer imaging. Nanotechnology 20:065102
Jayakumar R, Nair A, Rejinold NS, Maya S, Nair SV (2012) Doxorubicin-loaded pH-responsive chitin nanogels for drug delivery to cancer cells. Carbohydr Polym 87:2352–2356
Suzuki K, Mikami T, Okawa Y, Tokoro A, Suzuki S, Suzuki M (1986) Antitumor effect of hexa-N-acetylchitohex-aose and chitohexaose. Carbohydr Res 151:403–408
Copello GJ, Mebert AM, Raineri M, Pesenti MP, Diaz LE (2011) Removal of dyes from water using chitosan hydrogel/SiO2 and chitin hydrogel/SiO2 hybrid materials obtained by the sol–gel method. J Hazard Mater 186:932–939
Kousalya GN, Rajiv Gandhi M, Sairam Sundaram C, Meenakshi S (2010) Synthesis of nano-hydroxyapatite chitin/chitosan hybrid biocomposites for the removal of Fe(III). Carbohydr Polym 82:594–599
Rajiv Gandhi M, Kousalya GN, Meenakshi S (2011) Removal of copper(II) using chitin/chitosan nano-hydroxyapatite composite. Int J Biolog Macromol 48:119–124
Pinto PX, Al-Abed SR, Reisman DJ (2011) Biosorption of heavy metals from mining influenced water onto chitin products. Chem Eng J 166:1002–1009
Tang H, Chang C, Zhang L (2011) Efficient adsorption of Hg2+ ions on chitin/cellulose composite membranes prepared via environmentally friendly pathway. Chem Eng J 173:689–697
Tang H, Zhou W, Zhang L (2012) Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J Hazard Mater 209–210:218–225
Fan Y, Fukuzumi H, Saito T, Isogai A (2012) Comparative characterization of aqueous dispersions and cast films of different chitin nanowhiskers/nanofibers. Int J Biolog Macromol 50:69–76
Tong Y, Guan H, Wang S, Xu J, He C (2011) Syntheses of chitin-based imprinting polymers and their binding properties for cholesterol. Carbohydr Res 346:495–500
Dudarev VG, Iozep AA (2010) Synthesis of carboxymethyl chitin benzylidenehydrazides. Russ J Appl Chem 83:1853–1856
Acknowledgments
The authors would like to thank all the researchers whose published articles are used in this review for their invaluable information, investigation and examination in the field of chitin. Financial support of Iran National Science Foundation (INSF), under grant number: BN008 is also highly appreciated and acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barikani, M., Oliaei, E., Seddiqi, H. et al. Preparation and application of chitin and its derivatives: a review. Iran Polym J 23, 307–326 (2014). https://doi.org/10.1007/s13726-014-0225-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-014-0225-z