Abstract
Lignin is the main natural aromatic polymer and consists of about a quarter of lignocellulosic biomass. That is why products obtained from lignin are very attractive research topics, but it is also a very complex issue due to its complicated structure which depends on the separation method and plant species. To become a widely used raw material, the basic characteristics and structure-dependent properties must be elucidated, initially. For this reason, it is necessary to obtain lignin with superior properties, to be able to compete with fossil resources. This paper presents a chemical method to modify lignin by hydroxymethylation to obtain nanoparticles. Nanotechnology allows using chemical, physical and biological effects that do not occur outside the nanoscale world. To find the best conditions (from the average particle size distribution point of view), three reaction parameters were varied: ratio of lignin/aldehyde, pH and temperature. The following output value has been the average particle size distribution. The obtained data were processed with software in demo version (Modde 9) and resulted regression equation allows us to establish the optimum conditions. At the same time, the reaction products thus synthesized were characterized by FTIR spectroscopy, GPC, and 31PNMR spectroscopy. The results confirm that using this reaction, it is possible to synthesize nanoparticles from hydroxymethylated lignin. Lignin derivatives containing high hydroxyl group content have a potential utilization as phenol substitute in the phenol formaldehyde resin synthesis, composites, biocides, etc.
Similar content being viewed by others
Introduction
Nowadays, conventional raw material resources are limited and become difficult to assure. Therefore, renewable resources play an important role in the sustainable development concept. Lignin is a biopolymer, the main aromatic component in vegetal biomass, and it is the second most abundant component of lignocellulosic biomass [1–3] (about 28–30 % lignin content for softwood [4], 20–28 % for hardwood [5], 15–21 % for annual plants [6, 7]) after cellulose. The reactivity of lignin is determined by its origin and particular structure and also by the chemical structural modifications induced by separation methods.
At the same time, the reactivity of lignin can be increased using different reactions such as hydroxymethylation [8], epoxidation [9], carboxymethylation [10], esterification [11] oxidation and sulfonation [12], a.s.o. The main advantage of the hydroxymethylated lignin is its high content of hydroxyl groups which allows using it as phenol substitute in phenol formaldehyde resin synthesis [13], composites, biocides systems and bioremediation [14–16]. For instance, when lignin is hydroxymethylated, the resulting product is reported to replace up to 50 % of phenol in the phenol formaldehyde resins, without loss of properties [13, 17].
By modification of the parameters of hydroxymethylation reaction (time, temperature, catalyst type, formaldehyde/phenol ratio), lignin derivatives with different characteristics can be obtained. Thus, Schilling developed a method of obtaining a submicron (about 300 Å) lignin-based binder resins for flexographic water-borne black ink formulation using hydroxymethylation reaction [18].
Nanodispersions based on organic compounds, compared with other substances are not persistent in the environment. These nanodispersions can be used to achieve: biocides, pharmaceuticals/antioxidants, plant bioregulators, paints, etc. [14, 16, 18]. Taking into account the method proposed by Schilling, we carried out a study to establish the most convenient conditions for the synthesis of micro- and nano-scale particle size. The obtained lignin derivatives were characterized by FTIR spectroscopy, gel permeation chromatography and 31P NMR spectroscopy techniques and the resulted modifications were confirmed, as well.
Experimental
Materials
Sarkanda grass lignin (L2) obtained by an alkaline procedure of delignification was supplied by Granit Recherché Development Co., Switzerland. The characteristics of lignin (1.05-OHtotal groups/C9; 0.91-OHphenolic/C9; 0.96-OCH3/C9; 0.88-alkyl/aryl ratio; 0.88-carbonyl/C9; 0.82-syringyl/guaiacyl ratio) were determined in previous work [8]. All the other materials used were reagent grade.
Methods
A glass reactor equipped with a mechanical stirrer was immersed in a water bath. A quantity of 10 g of lignin (absolutely dry) was suspended in 47 mL of distilled water and stirred for 2 h at room temperature. After that, the heating was started to the set temperature. The reaction time was kept constant at 4 h and the temperature was varied between 50 and 95 °C. After the lignin suspension reached the desired temperature, a 50 % sodium hydroxide solution and an ammonia hydroxide (25 %) solution (depending on pH) were added and stirred for another 2 h. The pH of the mixture was varied in the range of 7.5–12.0 during the experiments. After 2 h of stirring, a formaldehyde solution (37 %) (depending on lignin/formaldehyde mass ratio) was added to the reaction mixture. The lignin/aldehyde ratio was varied between 0.5 and 1.5. A central composite factorial (CCF) experimental design was used to study the dependence of the particle size on varied reaction parameters. The obtained product was recovered by precipitation at pH 2 with a solution of hydrochloric acid (2 %), separated by centrifugation, washed and dried.
Characterization
Particle size distribution analysis of a suspension in distilled water of the modified lignin nanoparticles was carried out using a Shimadzu SALD 7001 (Japan) particle size analyzer. A single light source of violet laser combined with a single optical system allows the size determination of particles from 15 nm to 500 μm by laser diffraction method.
FTIR spectra were recorded with a Digilab Scimitar FTS2000 (USA) spectrophotometer using the KBr pellet method. The data acquisition conditions were: spectral width of 4,000–400 cm−1, 64 scans at a resolution of 4 cm−1. The pellet was prepared with a mixture of 200 mg KBr and 2 mg of lignin sample. FTIR data were processed using ACD Labs software.
Gel permeation chromatography was performed using a Shimadzu LC 20AT (Japan) liquid chromatograph with a SPD M20A ultraviolet diode array (UV) detector set at 280 nm. Two columns connected in series were used: Agilent PL gel MIXED-D 5 μm, 1–40 K and PL gel 5 μm, MW range 500 Da–20 kDa. Complete calibration was achieved using polystyrene standards provided by Fluka (Switzerland). THF was used as eluent, at flow rate of 0.5 mL/min. Before the injection, the lignin samples were acetylated with acetyl bromide following the procedure described by Lu and Ralph [19].
In the second GPC analysis variant, non-acetylated lignin samples analysis was carried out using TSK-gel GMPWxl GPC column (300 × 7, 8 mm, 500 Da–8,000 kDa). Elutions were performed with a pH 11 phosphate buffer solution, at a flow rate of 0.5 mL/min. Calibration curve was obtained using standards of poly(styrene sulfonic acid) sodium salt, provided by Fluka (Switzerland).
For 31PNMR, the lignin samples were derivatized with 2-chloro-4,4′,5,5′-tetramethyl-1,3,2-dioxaphospholane. Samples were dissolved in a solvent (1.6/1: pyridine/chloroform ratio) [20]. The 31P NMR spectra were performed on a Bruker 300 NMR (Germany) spectrometer. The NMR spectra were performed by Bruker Topspin software.
Results and discussion
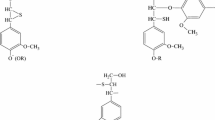
Formaldehyde reacts with lignin in the presence of alkali both by substituting the free 5 position in the phenolic (guaiacyl) nuclei, and by Tollens reaction of the side chains bearing carbonyl groups (Scheme 1). This reaction may be continued with a condensation resulting in the decrease of hydroxyl group content [21, 22].
During the hydroxymethylation process, formaldehyde is added to lignin in alkaline medium. Three reactions can take place. The main one is Lederer–Manasse reaction, where hydroxymethyl groups are incorporated to the lignin aromatic rings (Scheme 1, reaction a), increasing the reactivity of the molecule. Nondesirable reactions are the Cannizzaro reaction, in which formaldehyde reacts with itself, and Tollens reaction in which lignin side chains are substituted by aliphatic methylol groups (Scheme 1, reaction b). Increasing temperature, hydroxymethyl groups react at free positions of other lignin units or phenol to form methylene bonds (Scheme 1, reaction c). The reactivity of lignins in hydroxymethylation or cross-linking depends on its source (softwood, hardwood or grass) on the pulping conditions (pH, temperature, pressure) and on the reaction conditions.
Optimization of reaction conditions
The lignin separated from Sarkanda grass by an alkaline procedure was hydroxymethylated in different conditions (Table 1). With the aim to establish optimum conditions to synthesize nanoparticles based on hydroxymethylation of lignin, the following parameters were considered: the lignin/aldehyde mass ratio, pH and temperature. The dependent variable was the average particle size. The obtained data were used to optimize the hydroxymethylation reaction conditions and to determine the correlation equation between parameters and particle size. A demo version of adequate modeling software was used.
Response surface methodology can be employed to explore the contribution of a given set of independent variables (process parameters or system inputs) on a set of dependent ones (process results or system outputs). The coefficient of determination, R 2, designates the fitness between practical results and mathematical model. To determine the model predictive power, the parameter Q 2 is calculated. This reflects the validation of prediction. Parameter Q 2 is the fraction of the variation of the response predicted by the model according to cross validation and expressed in the same units as R 2. These parameters are used together as diagnostic tool for the model. Values close to 1 are desirable, because they indicate an excellent model. In practice, a Q 2 value higher than 0.5 designates a good model.
The difference between R 2 and Q 2 should not to be more than 0.2–0.3. The validation of prediction may be estimated using Eq. (1):
where, SStot is the total sum of the squares of the difference of the dependent variable and its grand mean; SSpred is the prediction residual sum of squares, computed by squared difference between observed dependent variable Y and predicted dependent variable Y [23].
The multiple linear regression (MLR) fitting method was used to validate experimental data and to generate a mathematical equation which could be used for optimization or for forecast results of hydroxymethylation reaction.
Based on statistical analysis results (p < 0.05; F > 1), a particular form (a second-degree polynomial) for the dependence of a system response as function of selected independent variables was found as described by Eq. (2). Thus, it was possible to obtain equation coefficients that describe the mathematical model (Table 2) as follows:
where z, average particle size; x, lignin/formaldehyde mass ratio; y, pH; T, temperature.
In Fig. 1 are represented, for example, average particle size distributions for some samples that resulted in different conditions of reactions (experiments 1, 2, 3, 4, 5, and 7) as compared to that of control sample (6). From this data, we can appreciate that by hydroxymethylation it is possible to obtain nanosized lignin particles. The Eq. (2) was used to study the influence of pH and lignin/formaldehyde ratio (L/A) for temperatures of 50, 72.5 and 90 °C (Figs. 2, 3, 4). From this graphical representation, the optimum conditions to obtain a convenient particle size distribution were established as: temperature = 72 °C, pH = 9.8, and lignin/formaldehyde ratio = 1.
Average particle size distribution analysis; samples 1, 2, 3, 4, 5, 7 (according to the Table 1) and sample 6 as control sample
Characterization of reaction products
FTIR analysis
FTIR spectroscopy was used to characterize the raw material and to analyze the changes in the lignin structure during the hydroxymethylation (Table 3) [24–26]. The FTIR spectra obtained are characterized by a broad O–H band at 3,400 cm−1 (which were increased in the case of modified samples), an intense C–H band at 2,927 cm−1, and another at 2,854 cm−1, typical of methoxyl groups and also hydroxymethyl groups. The absorbance values at these wavenumbers were increased for hydroxymethylated samples. The aromatic skeletal vibration occurs at 1,600 and 1,500 cm−1. The band at 1,600 cm−1 was used for normalization and its intensity was always set to 1.00. The C–H deformations of asymmetric methyl and methylene appeared at 1,470–1,460 cm−1, and the ether carbon–oxygen bands appeared at 1,400–1,000 cm−1. The band at 1,370 cm−1 was due to the bending vibration of the phenolic OH group. Bands at 1,140 and 1,035 cm−1 are characteristic to secondary and primary OH groups, respectively [27]. Also, a band at 836 cm−1, which is due to the aromatic C–H out of plane vibration in p-hydroxyphenyl propane, was also detected (Fig. 5). More exact and complete information was provided by 31P NMR spectroscopy technique.
GPC analysis
It is known that lignin molecular mass and its distribution are parameters related to the physico-chemical properties which are very important in the development of potential applications. Molecular mass distribution may be used to monitor the modification of lignin during performed physico-chemical treatments [28].
After reaction, the resulted hydroxymethylated samples along with non-modified lignin were characterized by GPC using non-acetylated and acetylated derivatives. The main advantages of gel permeation chromatography are accessibility, short time of analysis, small quantities of sample and the possibility of determining the molecular mass on a very large scale. The results obtained from GPC are presented in Figs 6 and 7 and Tables 4 and 5. Both applied variants evidenced that hydroxymethylation of lignin took place by modification of average macromolecular mass. According to calculation of the values obtained for Sarkanda grass lignin (L2) and two hydroxymethylated samples (selected from those corresponding from dimensional point of view, Ex4, Ex7) was observed a decrease of the weight average molecular mass (M w) and at the same time an increase of the number average molecular mass (M n) for both types of analysis. These data confirm the results reported in a previous work [13]. Thus, the polydispersity index (PID) decrease proved that by hydroxymethylation, a nanostructured polymer with a more uniform molecular mass can be obtained.
However, there are some differences between the values resulted using the two techniques. These can be explained by association phenomena by hydrogen bonds including fragments of lignin with different molecular mass of non-acetylated samples, which are characterized by lower values. In case of acetylated lignin derivatives, the hydroxyl groups were blocked, preventing molecular association and thus the values of molecular mass and polydispersity indices were higher.
31P NMR analysis
NMR spectroscopy is a useful technique to observe the evolution of functional groups during lignin modification. The use of phosphorous-containing derivatizing reagents for lignin analysis has grown in importance. The sensitivity of a 31PNMR experiment is about 15 times less than that of a proton NMR experiment. The range of 31P chemical shifts is more than 1,000 ppm for a variety of phosphorous compounds and the average line width is about 0.7 Hz [29]. This technique was used to characterize non-modified and hydroxymethylated lignin samples (Fig. 8; Table 6) after derivatizing them with phosphorous compounds. The results obtained allow to distinguish the different types of functional groups which can be quantified by integrating the following regions for: aliphatic between 150.8 and 146.3 ppm, condensed phenolic units (diphenylmethane type) between 144.3 and 142.8 ppm, syringyl phenolic units between 143.7 and 142.2 ppm, condensed phenolic units (4-O-5′ type) between 142.8 and 141.7 ppm, condensed phenolic units (5-5′ type) between 141.7 and 140.2 ppm, guaiacyl and demethylated phenolic units between 140.2 and 138.4 ppm, p-hydroxyphenolic units between 138.6 and 136.9 ppm and carboxylic acids between 135.6 and 133.7 [30–32].
After the hydroxymethylation reaction, spectra integration for unmodified Sarkanda grass lignin (L2) and modified by hydroxymethylation (samples Ex 4, Ex7), it was observed that the peak area in chemical shift range 140–137 ppm decreases simultaneously with the increasing of the peak area in 144,3–142,8 ppm chemical shift. Thus, the introduction of hydroxymethyl groups in lignin macromolecules was confirmed by 31PNMR spectra analysis and also further condensation with diphenylmethane bonds formation (Table 6).
Conclusion
Using hydroxymethylation reaction of lignin, it was possible to obtain nanoparticles and by mathematical modeling optimum reaction conditions were established as being: temperature = 72 °C, pH = 9.8, lignin/formaldehyde ratio = 1. The nanoparticles were characterized from dimensional point of view. The modifications determined by hydroxymethylation reaction were confirmed by FTIR spectroscopy, GPC-chromatography and 31P-NMR spectroscopy techniques. All of the performed analysis show that use of the proposed chemical method lead to hydroxymethylated lignin nanoparticles.
References
Dimmel D (2010) Overview. In: Heitner C, Dimmel D, Schmidt J (eds) Lignin and lignans: advances in chemistry, chap 1. CRC Press, Boca Raton, pp 1–10
Xing W, Yuan H, Zhang P, Yang H, Song L, Hu Y (2013) Functionalized lignin for halogen-free retardant rigid polyurethan fooam: preparation, thermal stability, fire performance and mechanical properties. J Polym Res 20:234–244
Sarkanen KV, Ludwing CH (1971) In: Sarkanen KV, Ludwing CH (eds) Lignins: occurence, formation, structural and reaction. Wiley, New York
Petrovici G, Popa VI (1997) Chimia si prelucrarea chimica a lemnului (in Romanian). Lux Libris, Brasov
Pinkert A, Goeke DF, Marsh KN, Pang S (2011) Extracting wood lignin without dizolving or degrading cellulose: investigations on the use of food additive-derived ionic liquids. Green Chem 13:3124–3136
del Rio JC, Rencoret J, Gutierrez A, Nieto L, Barbero JJ, Matrinez AT (2011) Structural characterization of guaiacyl-rich lignins in flax (Linum usitatissimum) fibers and shives. J Agric Food Chem 59:11088–11099
Sarkanen KV, Herget HL (1971) In Sarkanen KV, Ludwing CH (eds) Lignins: occurence, formation, structural and reaction. Wiley-Interscience, New York
Capraru AM, Ungureanu E, Trinca L, Malutan T, Popa VI (2012) Chemical and spectral characteristics of annual plant lignins modified by hydroxymethylation reaction. Cell Chem Technol 46:589–597
Malutan T, Nicu R, Popa VI (2008) Lignin modification by epoxidation. BioResources 3:1371–1376
Gilca IA, Capraru AM, Popa VI (2011) Studies concerning the obtaining and characterization of lignophenols derivatives. Bull Polytech Inst Iasi 57:141–147
Maldhure A, Chaudhari AR, Ekhe JD (2011) Thermal and structural studies polypropylene blended with esterified industrial waste lignin. J Therm Anal Calorim 103:625–632
Xiang Y, Xu W, Ou E, Su Q, Chen L, Zhan Y, Xia X, Xiong Y, Xiong Y (2013) Preparation and characterization of strongly swellable modified-lignosulfonate hydrogel particles. Iran Polym J 22:749–756
Malutan T, Nicu R, Popa VI (2008) Contribution to the study of hydroxymethylation reaction of alkali lignin. BioResources 3:13–20
Popa VI, Capraru AM, Grama S, Malutan T (2011) Nanoparticles based on modified lignins with biocide properties. Cell Chem Technol 45:221–226
Gilca IA, Capraru AM, Grama S, Popa VI (2011) Agents for wood bioprotection based on natural aromatic compounds and their complexes with copper and zinc. Cell Chem Technol 45:227–231
Gilca IA, Popa VI (2013) Study on biocidal properties of some nanoparticles based on epoxy lignin. Cell Chem Technol 47:239–245
Hu L, Pan H, Zhou Y, Zhang M (2011) Methods to improve lignin’s reactivity as a phenol substitute and as a replacement for other phenolic compounds: a brief review. BioResources 6:3515–3525
Schilling P (1993) Submicron lignin-based binders for water-based black ink formulations. US Patent number 5192361
Lu F, Ralph J (1998) The DFRC method for lignin analysis. Part 3. NMR studies. J Wood Chem Technol 18:219–233
Argyropoulos DS (1994) Quantitative phosphouros-31 NMR analysis of six soluble lignins. J Wood Chem Technol 14:65–82
Bernar P, Goncalves AR, Mandelli D, Ferreira MMC, Schuchart U (1999) Principal component analysis of the hydroxymethylation of sugarcane lignin: a time depending study by FTIR. J Wood Chem Technol 19:151–165
Bernar P, Goncalves AR, Mandelli D, Schuchart U (1999) Eucalyptus organosolv lignins: study of the hydroxymethylation and use in resols. Bioresour Technol 68:11–16
Eriksson L, Johansson E, Kettaneh-Wold N, Wikstrom C, Woold S (2008) Design of experiments: principles and applications. Umetrics AB, Umea
Alonso MV, Rodriguez JJ, Oliet M, Rodriguez F, Garcia J, Gilarranz MA (2001) Characterization and structural modification of ammonic lignosulfonate by methylation. J Appl Polym Sci 82:2661–2668
Gilli E, Schmied F, Diebald S, Horvath AT, Teichert C, Schennach R (2012) Analysis of lignin precipitates on ozone treated kraft pulp by FTIR and AFM. Cellulose 19:249–256
Kang S, Xiao L, Meng L, Zhang X, Sun R (2012) Isolation and structural characterization of lignin from cotton stalk treated in an ammonia hydrothermal system. Int J Mol Sci 13:15209–15226
Nada AAMA, El-Sakhawy M, Kamel SM (1998) Infra-red specroscopic study of lignins. Polym Degrad Stab 60:247–251
Gosselink RJA (2011) Lignin as a renewable aromatic resource for the chemical industry. PhD Thesis, Wageningen University, Wageningen, The Netherlands
Argyropoulos DS (2010) Heteronuclear NMR spectroscopy of lignins. In: Heitner C, Dimmel D, Schmidt J (eds) Lignin and lignans: advances in chemistry. chap 6, CRC Press, Boca Raton, pp 245–265
Crestini C, Argyropoulos DS (1997) Structural analysis of wheat straw lignin by quantitative 31P and 2D NMR spectroscopy. The occurrence of ester bonds and α-O-4 substructures. J Agric Food Chem 45:1212–1219
Granata A, Argyropoulos DS (1995) 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, a reagent for the accurate determination of the uncondensed and condensed phenolic moieties and lignin. J Agric Food Chem 43:1538–1544
Argyropoulos DS, Bolker HI, Hetner C, Archipov Y (1993) 31P NMR spectroscopy in wood chemistry. Part V. Quantitative analysis of lignin functional groups. J Wood Chem Technol 13:187–212
Acknowledgments
This paper was realised with the support of POSDRU CUANTUMDOC “DOCTORAL STUDIES FOR EUROPEAN PERFORMANCES IN RESEARCH AND INNOVATION” ID79407 project funded by the European Social Found and Romania Government. The authors are grateful to Dr. Claudia Crestini and Dr. Federica Melone from University of Rome “Tor Vergata” for support in GPC acetylated and 31P-NMR analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gilca, I.A., Ghitescu, R.E., Puitel, A.C. et al. Preparation of lignin nanoparticles by chemical modification. Iran Polym J 23, 355–363 (2014). https://doi.org/10.1007/s13726-014-0232-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-014-0232-0