Abstract
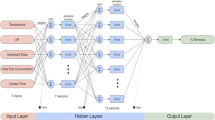
Metal-complex dyes are widely used in textile industry, but harmful to the environment and human health due to aromatic structure and heavy metal ions. The objective of this work was to evaluate the adsorption potential of bamboo biochar for the removal of metal-complex dye acid black 172 from solutions. Freundlich model was more suitable for the adsorption process of bamboo biochar than Langmuir isotherm, indicating multilayer adsorption of acid black 172 on a heterogeneous bamboo biochar surface. Adsorption kinetics analysis of pseudo-second-order and Weber–Morris models revealed that intraparticle transport was not the only rate-limiting step. The bamboo biochar exhibited a good adsorption performance even at high ionic strength. Analysis based on the artificial neural network indicated that the temperature with a relative importance of 29 % appeared to be the most influential parameter in the adsorption process for dye removal, followed by time, ionic strength, pH and dye concentration.






Similar content being viewed by others
References
Aksu Z, KarabayIr G (2008) Comparison of biosorption properties of different kinds of fungi for the removal of Gryfalan Black RL metal-complex dye. Bioresource Technol 99(16):7730–7741
Al-Degs YS, El-Barghouthi MI, El-Sheikh AH, Walker GM (2008) Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigm 77(1):16–23
Çelekli A, Birecikligil SS, Geyik F, Bozkurt H (2012) Prediction of removal efficiency of Lanaset Red G on walnut husk using artificial neural network model. Bioresource Technol 103(1):64–70
Chen DZ, Zhang JX, Chen JM (2010) Adsorption of methyl tert-butyl ether using granular activated carbon: equilibrium and kinetic analysis. Int J Environ Sci Technol 7(2):235–242
Chiou MS, Li HY (2003) Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 50(8):1095–1105
Debrassi A, Baccarin T, Demarchi CA, Nedelko N, Slawska-Waniewska A, Dluzewski P, Bilska M, Rodrigues CA (2012) Adsorption of Remazol Red 198 onto magnetic N-lauryl chitosan particles: equilibrium, kinetics, reuse and factorial design. Environ Sci Pollut R 19(5):1594–1604
Demirbas A (2009) Agricultural based activated carbons for the removal of dyes from aqueous solutions: a review. J Hazard Mater 167(1–3):1–9
Forgacs E, Cserháti T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30(7):953–971
Freundlich HMF (1906) Über die adsorption in lösungen. Z Phys Chem 57:385–470
Garson GD (1991) Interpreting neural-network connection weights. AI Expert 6(4):46–51
Hameed BH, El-Khaiary MI (2008) Kinetics and equilibrium studies of malachite green adsorption on rice straw-derived char. J Hazard Mater 153(1–2):701–708
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf Environ Prot 76(B2):183–191
Itodo AU, Abdulrahman FW, Hassan LG, Maigandi SA, Itodo HU (2010) Intraparticle diffusion and intraparticulate diffusivities of herbicide on derived activated carbon. Researcher 2(2):74–86
Jindarom C, Meeyoo V, Kitiyanan B, Rirksomboon T, Rangsunvigit P (2007) Surface characterization and dye adsorptive capacities of char obtained from pyrolysis/gasification of sewage sludge. Chem Eng J 133(1–3):239–246
Khataee AR, Dehghan G, Ebadi A, Zarei M, Pourhassan M (2010) Biological treatment of a dye solution by Macroalgae Chara sp.: effect of operational parameters, intermediates identification and artificial neural network modeling. Bioresource Technol 101(7):2252–2258
Kumar S, Zafar M, Prajapati JK, Kumar S, Kannepalli S (2011) Modeling studies on simultaneous adsorption of phenol and resorcinol onto granular activated carbon from simulated aqueous solution. J Hazard Mater 185(1):287–294
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Liu Y, Zhao X, Li J, Ma D, Han R (2012) Characterization of bio-char from pyrolysis of wheat straw and its evaluation on methylene blue adsorption. Desalination Water Treat 46(1–3):115–123
Mahmoud DK, Salleh MAM, Karim W, Idris A, Abidin ZZ (2012) Batch adsorption of basic dye using acid treated kenaf fibre char: equilibrium, kinetic and thermodynamic studies. Chem Eng J 181:449–457
Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 11(2):431–441
Maurya NS, Mittal AK, Cornel P, Rother E (2006) Biosorption of dyes using dead macro fungi: effect of dye structure, ionic strength and pH. Bioresource Technol 97(3):512–521
Mittal A, Thakur V, Gajbe V (2012) Evaluation of adsorption characteristics of an anionic azo dye Brilliant Yellow onto hen feathers in aqueous solutions. Environ Sci Pollut R 19(6):2438–2447
Mui ELK, Cheung WH, McKay G (2010a) Tyre char preparation from waste tyre rubber for dye removal from effluents. J Hazard Mater 175(1–3):151–158
Mui ELK, Cheung WH, Valix M, McKay G (2010b) Dye adsorption onto char from bamboo. J Hazard Mater 177(1–3):1001–1005
Özer A, Akkaya G, Turabik M (2006) Biosorption of Acid Blue 290 (AB 290) and Acid Blue 324 (AB 324) dyes on Spirogyra rhizopus. J Hazard Mater 135(1–3):355–364
Park D, Yun YS, Park JM (2010) The past, present, and future trends of biosorption. Biotechnol Bioprocess Eng 15(1):86–102
Qiu YP, Zheng ZZ, Zhou ZL, Sheng GD (2009) Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresource Technol 100(21):5348–5351
Safarikova M, Ptackova L, Kibrikova I, Safarik I (2005) Biosorption of water-soluble dyes on magnetically modified Saccharomyces cerevisiae subsp uvarum cells. Chemosphere 59(6):831–835
Shen DZ, Fan JX, Zhou WZ, Gao BY, Yue QY, Kang Q (2009) Adsorption kinetics and isotherm of anionic dyes onto organo-bentonite from single and multisolute systems. J Hazard Mater 172(1):99–107
Subramanyam B, Das A (2009) Linearized and non-linearized isotherm models comparative study on adsorption of aqueous phenol solution in soil. Int J Environ Sci Technol 6(4):633–640
Vijayaraghavan K, Han MH, Choi SB, Yun YS (2007) Biosorption of Reactive black 5 by Corynebacterium glutamicum biomass immobilized in alginate and polysulfone matrices. Chemosphere 68(10):1838–1845
Wang L-G, Yan G-B (2011) Adsorptive removal of direct yellow 161dye from aqueous solution using bamboo charcoals activated with different chemicals. Desalination 274(1–3):81–90
Xiong X-J, Meng X-J, Zheng T-L (2010) Biosorption of C.I. Direct Blue 199 from aqueous solution by nonviable Aspergillus niger. J Hazard Mater 175(1–3):241–246
Xu RK, Xiao SC, Yuan JH, Zhao AZ (2011) Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues. Bioresource Technol 102(22):10293–10298
Yang Y, Wang G, Wang B, Li Z, Jia X, Zhou Q, Zhao Y (2011) Biosorption of Acid Black 172 and Congo Red from aqueous solution by nonviable Penicillium YW 01: kinetic study, equilibrium isotherm and artificial neural network modeling. Bioresource Technol 102(2):828–834
Yang YY, Li ZL, Wang G, Zhao XP, Crowley DE, Zhao YH (2012) Computational identification and analysis of the key biosorbent characteristics for the biosorption process of Reactive Black 5 onto fungal biomass. PLoS ONE 7(3):e33551
Zarei M, Niaei A, Salari D, Khataee AR (2010) Removal of four dyes from aqueous medium by the peroxi-coagulation method using carbon nanotube-PTFE cathode and neural network modeling. J Electroanal Chem 639(1–2):167–174
Acknowledgments
This work was supported in part by Grants from the Science and Technology Project of Zhejiang Province (2010C13G2010074), National Natural Science Foundation of China (31070079), the Science and Technology Project of Zhejiang Province (2008C13014-3), the International Cooperation Project in Science and Technology of Zhejiang Province (No. 2008C14038), the National Key Technology Rand D Program (2012BAC17B04), and Open Funding Project of the Key Laboratory of Aquatic Botany and Watershed Ecology, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Lin, X., Wei, B. et al. Evaluation of adsorption potential of bamboo biochar for metal-complex dye: equilibrium, kinetics and artificial neural network modeling. Int. J. Environ. Sci. Technol. 11, 1093–1100 (2014). https://doi.org/10.1007/s13762-013-0306-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-013-0306-0