Abstract
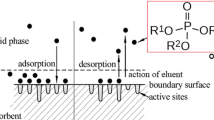
There are compelling economic and environmental reasons to remove pesticides from wastewater because they are toxic and carcinogenic. The effectiveness of copper-based metal–organic framework (Cu-BTC) for adsorbing the insecticide 14C-ethion from wastewater has been studied as function of contact time, adsorbent dosage, temperature and pH. 14C-ethion/Cu-BTC isotherms exhibit two plateaus (BET type IV) and are reliably represented by Brunauer–Deming–Deming–Teller and Zhu–Gu models, with deviations of only 1.99 and 3.95%, respectively. The removal curve measured under batch operation is well represented by a pseudo-first-order equation, yielding results equivalent to the theoretical linear driving force model of Glueckauf. At pH 7, 75 mg L−1 ethion concentration, 150 min, 25 °C and 0.425 g L−1 Cu-BTC dose, the sorbent capacity is ca. 122 mg g−1. Moreover, Cu-BTC has a good stability after six adsorptions cycles. Finally, our results disclose the fundamental understanding of the adsorption mechanism: the ethion molecule coordinates to two copper(II) atoms across the metal–organic framework channel via the phosphoryl (P–O) group.










Similar content being viewed by others
References
Abdel-Gawad H, Abdelhameed RM, Elmesalamy AM, Hegazi B (2011) Distribution and elimination of 14C-ethion insecticide in chamomile flowers and oil. Phosphorus Sulfur Silicon 186:2122–2134. doi:10.1080/10426507.2011.588506
Abdelhameed RM, Abdel-Gawad H, Elshahat M, Emam HE (2016) Cu–BTC@cotton composite: design and removal of ethion insecticide from water. RSC Adv 6:42324–42333. doi:10.1039/C6RA04719J
Abdelhameed RM, Emam HE, Rocha J, Silva AMS (2017) Cu-BTC metal–organic framework natural fabrics composites for fuel purification. Fuel Process Technol 159:306–312. doi:10.1016/j.fuproc.2017.02.001
Ahmad T, Rafatullah M, Ghazali A, Sulaiman O, Hashim R, Ahmad A (2010) Removal of pesticides from water and wastewater by different adsorbents: a review. J Environ Sci Health Part C 28:231–271. doi:10.1080/10590501.2010.525782
Aniceto JPS, Silva CM (2015) Preparative chromatography: batch and continuous. In: Anderson JL, Berthod A, Pino V, Stalcup A (eds) Analytical separation science, vol 5, Chapter 4. Wiley, New York, pp 1207–1313
Asfaram A, Ghaedi M, Agarwal S (2015) Removal of basic dye Auramine-O by ZnS:Cu nanoparticles loaded on activated carbon: optimization of parameters using response surface. RSC Adv 5:18438–18450. doi:10.1039/C4RA15637D
Bae G-T, Dellinger B, Hall RW (2011) Density functional calculation of the structure and electronic properties of CunOn (n = 1–8) clusters. J Phys Chem A 115:2087–2095. doi:10.1021/jp104177q
Brunauer S, Deming LS, Deming WE, Teller E (1940) On a theory of the van der waals adsorption of gases. J Am Chem Soc 62:1723–1732. doi:10.1021/ja01864a025
Danmaliki GI, Saleh TA (2017) Effects of bimetallic Ce/Fe nanoparticles on the desulfurization of thiophenes using activated carbon. Chem Eng J 307:914–927. doi:10.1016/j.cej.2016.08.143
Danmaliki GI, Saleh TA, Shamsuddeen AA (2017) Response surface methodology optimization of adsorptive desulfurization on nickel/activated carbon. Chem Eng J 313:993–1003. doi:10.1016/j.cej.2016.10.141
Dehghani MH, Niasar ZS, Mehrnia MR, Shayeghi M, Al-Ghouti MA, Heibati B, McKay G, Yetilmezsoy K (2017) Optimizing the removal of organophosphorus pesticide malathion from water using multi-walled carbon nanotubes. Chem Eng J 310:22–32. doi:10.1016/j.cej.2016.10.057
Do DD (1998) Adsorption analysis: equilibrium kinetics. Imperial College Press, London
Ebadi A, Mohammadzadeh JSS, Khudiev A (2009) What is the correct form of BET isotherm for modeling liquid phase adsorption? Adsorption 15:65–73. doi:10.1007/s10450-009-9151-3
Elwakeel KZ, Yousif AM (2010) Adsorption of malathion on thermally treated egg shell material. Water Sci Technol 61:1035–1041. doi:10.2166/wst.2010.005
Foster LJR, Kwan BH, Vancov T (2004) Microbial degradation of the organophosphate pesticide, Ethion. FEMS Microbiol Lett 240(1):49–53. doi:10.1016/j.femsle.2004.09.010
Girods P, Dufour A, Fierro V, Rogaume Y, Rogaume C, Zoulalian A, Celzard A (2009) Activated carbons prepared from wood particleboard wastes: characterization and phenol adsorption capacities. J Hazard Mater 166:491–501. doi:10.1016/j.jhazmat.2008.11.047
Glueckauf E (1955) Theory of chromatography—Part X. Formulae for diffusion into spheres and their application to chromatography. Trans Faraday Soc 51:1540–1551. doi:10.1039/TF9555101540
Glueckauf E, Coates J (1947) Theory of chromatography—part IV. The influence of incomplete equilibrium on the front boundary of chromatograms and on the effectiveness of separation. J Chem Soc 1315–1321. doi:10.1039/JR9470001315
Gnanasekaran L, Hemamalini R, Saravanan R, Ravichandran K, Gracia F, Agarwal S, Gupta VK (2017) Synthesis and characterization of metal oxides (CeO2, CuO, NiO, Mn3O4, SnO2 and ZnO) nanoparticles as photocatalysts for degradation of textile dyes. J Photochem Photobiol B Biol 173:43–49. doi:10.1016/j.jphotobiol.2017.05.027
Gupta VK, Jain C, Ali I, Chandra S, Agarwal S (2002) Removal of lindane and malathion from wastewater using bagasse fly ash—a sugar industry waste. Water Res 36:2483–2490. doi:10.1016/S0043-1354(01)00474-2
Gupta VK, Tyagi I, Agarwal S, Sadegh H, Shahryari-ghoshekandi R, Yari M, Yousefi-Nejat O (2015) Experimental study of surfaces of hydrogel polymers HEMA, HEMA–EEMA–MA, and PVA as adsorbent for removal of azo dyes from liquid phase. J Mol Liq 206:129–136. doi:10.1016/j.molliq.2015.02.015
Gupta VK, Saravanan R, Agarwal S, Gracia F, Khan MM, Qin J, Mangalaraja RV (2017) Degradation of azo dyes under different wavelengths of UV light with chitosan–SnO2 nanocomposites. J Mol Liq 232:423–430. doi:10.1016/j.molliq.2017.02.095
Habila MA, Alothman ZA, Al-Tamrah SA, Ghafar AA, Soylak M (2015) Activated carbon from waste as an efficient adsorbent for malathion for detection and removal purposes. J Ind Eng Chem 32:336–344. doi:10.1016/j.jiec.2015.09.009
Hasan Z, Jhung SH (2015) Removal of hazardous organics from water using metal–organic frameworks (MOFs): plausible mechanisms for selective adsorptions. J Hazardous Mater 283:329–339. doi:10.1016/j.jhazmat.2014.09.046
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. doi:10.1016/S0032-9592(98)00112-5
Hoffmann I, Oppel C, Gernert U, Barreleiro P, Von Rybinski W, Gradzielski M (2012) Adsorption isotherms of cellulose-based polymers onto cotton fibers determined by means of a direct method of fluorescence spectroscopy. Langmuir 28:7695–7703. doi:10.1021/la300192q
Huang MR, Peng QY, Li XG (2006) Rapid and effective adsorption of lead ions on fine poly(phenylenediamine) microparticles. Chem Eur J 12:4341–4350. doi:10.1002/chem.200501070
Khan NA, Hasan Z, Jhung SH (2013) Adsorptive removal of hazardous materials using metal–organic frameworks (MOFs): a review. J Hazard Mater 244:444–456. doi:10.1016/j.jhazmat.2012.11.011
Kumar P, Singh H, Kapur M, Mondal MK (2014) Comparative study of malathion removal from aqueous solution by agricultural and commercial adsorbents. J Water Process Eng 3:67–73. doi:10.1016/j.jwpe.2014.05.010
Largergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga svenska vetenskapsakademiens. Handlingar 24:1–39
Li H, Eddaoudi M, O’Keeffe M, Yaghi OM (1999) Design and synthesis of an exceptionally stable and highly porous metal–organic framework. Nature 402:276–279. doi:10.1038/46248
Liu J, Culp JT, Natesakhawat S, Bockrath BC, Zande B, Sankar S, Garberoglio G, Johnson JK (2007) Experimental and theoretical studies of gas adsorption in Cu3(BTC)2: an effective activation procedure. J Phys Chem C 111:9305–9313. doi:10.1021/jp071449i
Naushad M, Alothman Z, Khan M (2013) Removal of malathion from aqueous solution using De-Acidite FF-IP resin and determination by UPLC–MS/MS: equilibrium, kinetics and thermodynamics studies. Talanta 115:15–23. doi:10.1016/j.talanta.2013.04.015
Nile Basin Initiative—Nile basin national water quality monitoring baseline study report for Egypt (2005) Nile Basin Regional Water Quality Monitoring Baseline Study Report—Final
Qadir M, Mateo-Sagasta J, Jiménez B, Siebe C, Siemens J, Hanjra MA (2015) Environmental risks and cost-effective risk management in wastewater use systems. In: Drechsel P, Qadir M, Wichelns D (eds) Wastewater—economic asset in an urbanizing world. Springer, Netherlands, pp 55–72
Robati D, Mirza B, Rajabi M, Moradi O, Tyagi I, Agarwal S, Gupta VK (2016) Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem Eng J 284:687–697. doi:10.1016/j.cej.2015.08.131
Rodrigues AE, Silva CM (2016) What’s wrong with Lagergreen pseudo first order model for adsorption kinetics? Chem Eng J 306:1138–1142. doi:10.1016/j.cej.2016.08.055
Rowsell JL, Yaghi OM (2006) Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal–organic frameworks. J Am Chem Soc 128:1304–1315. doi:10.1021/ja056639q
Sainio T, Turku I (2010) Adsorption of cationic surfactants on a neutral polymer adsorbent: investigation of the interactions by using mathematical modeling. Coll Surf A 358:57–67. doi:10.1016/j.colsurfa.2010.01.031
Saleh TA, Sarı A, Tuzen M (2017) Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chem Engin J 307:230–238. doi:10.1016/j.cej.2016.08.070
Saravanan R, Sacari E, Gracia F, Khan MM, Mosquera E, Gupta VK (2016) Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. J Mol Liq 221:1029–1033. doi:10.1016/j.molliq.2016.06.074
Stavila V, Talin A, Allendorf M (2014) MOF-based electronic and opto-electronic devices. Chem Soc Rev 43:5994–6010. doi:10.1039/c4cs00096j
Tanner PA, Leung K-H (1996) Spectral interpretation and qualitative analysis of organophosphorus pesticides using ft-raman and ft-infrared spectroscopy. Appl Spectrosc 50:565–571
Vishnyakov A, Ravikovitch PI, Neimark AV, Bülow M, Wang QM (2003) Nanopore structure and sorption properties of Cu–BTC metal–organic framework. Nano Lett 3:713–718. doi:10.1021/nl0341281
Younis SA, Ghobashy MM, Samy M (2017) Development of aminated poly(glycidyl methacrylate) nanosorbent by green gamma radiation for phenol and malathion contaminated wastewater treatment. J Environ Chem Eng 5:2325–2336. doi:10.1016/j.jece.2017.04.024
Zhang T, Lin W (2014) Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem Soc Rev 43:5982–5993. doi:10.1039/C4CS00103F
Zhu B-Y, Gu T (1989) General isotherm equation for adsorption of surfactants at solid/liquid interfaces. Part 1. Theoretical. J Chem Soc Faraday Trans 1(85):3813–3817. doi:10.1039/F19898503813
Zhu B-Y, Gu T, Zhao X (1989) General isotherm equation for adsorption of surfactants at solid/liquid interfaces. Part 2. Applications. J Chem Soc Faraday Trans 1(85):3819–3824. doi:10.1039/F19898503819
Zhu X, Li B, Yang J, Li Y, Zhao W, Shi J, Gu J (2014) Effective adsorption and enhanced removal of organophosphorus pesticides from aqueous solution by Zr-based MOFs of UIO-67. ACS Appl Mater Interfaces 7:223–231. doi:10.1021/am5059074
Acknowledgements
The authors would like to thank the help of Prof. Dr. H. Kamel, Radioisotopes Department, Atomic Energy Authority, Cairo, Egypt, in the radioactivity measurements. This work was developed within the scope of the projects CICECO-Aveiro Institute of Materials (Ref. FCT UID/CTM/50011/2013) and UI QOPNA (Ref. FCT UID/QUI/00062/2013), financed by national funds through the FCT/MEC and when appropriate co-financed by FEDER under the PT2020 Partnership Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: V.K. Gupta.
Rights and permissions
About this article
Cite this article
Abdelhameed, R.M., Abdel-Gawad, H., Silva, C.M. et al. Kinetic and equilibrium studies on the removal of 14C-ethion residues from wastewater by copper-based metal–organic framework. Int. J. Environ. Sci. Technol. 15, 2283–2294 (2018). https://doi.org/10.1007/s13762-017-1624-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1624-4