Abstract
Magnetic nanoparticles iron oxide with average sizes of 6 nm were synthesized by a chemical coprecipitation method from mixtures of FeCl2·4H2O and FeCl3·6H2O. For preparation, multi-walled carbon nanotubes (MWCNTS) with outer diameter of 50 nm, wall thickness from 1 to 2 nm and length from 500–2,000 nm were used. Characterization of the MWCNT–Fe3O4 by X-ray powder diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, scanning electron microscope (SEM), transmission electron microscope (TEM), thermo-gravimetric analysis (TGA) and magnetic characterization was conducted on a vibrating sample magnetometer (VSM).
Similar content being viewed by others
Introduction
Since discovery of their structures in 1991 [1], carbon nanotubes (CNTs) have attracted considerable interdisciplinary interest because of their unique physical and chemical properties; however, the hydrophobicity of CNTs may limit their application [2, 3]. Therefore, surface modification of CNTs with functional groups and/or nanoparticles so as to disperse them into aqueous solutions becomes a key step for their applications [4].
Magnetic nanoparticles are gaining importance as they can be used as highly effective, efficient and economically-viable adsorbents, with the additional advantage of their easy separation under a magnetic field for reuse [5].
Herein, we describe a very simple, direct, and effective approach for the decoration of multi-walled carbon nanotubes (MWCNTs) by Fe3O4 magnetic nanoparticles. Our results suggest a novel and simple approach for controlling the size and size distribution of Fe3O4 on the surface of MWCNTs, which leads to a further development of CNT-based nanomaterials.
Experimental
Material
MWCNTs were purchased from NanoAmor Nanostructured & Amorphous Materials, Inc, USA [Purity >95 %, outer >50 nm, length 500–2,000 nm, surface area ~40m2/g, and manufacturing method catalytic chemical vapor deposition (CVD)]. Other chemicals were purchased from Merck Inc, USA.
Instrumentation
X-ray powder diffraction (XRD) analysis was conducted on a Rigaku Smart Lab Diffractometer operated at 40 kV and 35 mA using Cu Kα radiation.
The characteristics of the MWCNT-Fe3O4 were analyzed by attenuated total reflection-Fourier transform infrared spectrometer (100 spectra accumulation, 2 cm−1 resolution, BOMEM, Canada). FTIR samples were prepared by grinding dried MWCNTs and MWCNT-Fe3O4 together with potassium bromide (KBr) to make a pellet that was dried in an oven for 8 h before the test.
A morphological analysis was carried out using a scanning electron microscope (SEM), (TESCAN, VEGA 3, USA).
Transmission electron microscopy (TEM) analysis was performed using a JEOL JEM microscope (TEM, JEOL 2010, Japan) operating at 200 kV by depositing sample onto the lacey carbon-coated copper grids.
Thermo-gravimetric analysis (TGA) of all samples was done using SDT Q600 instrument from TA instrument. The temperature ranged from 25 to 1,000 °C and the heating rate was 10 °C/min.
Magnetic characterization was conducted on a vibrating sample magnetometer (VSM) (PPMS VSM, Model 6000).
Synthesis of MWCNT-Fe3O4
Iron oxide nanoparticles were firstly synthesized by chemical coprecipitation according to the method reported with a minor modification [6, 7].
Typically, FeCl2·4H2O and FeCl3·6H2O with a molar ratio of 1:2 were mixed in deionized water. Afterwards, 10 mL of 2 mol/L HCl was added. The solution was heated to 30 °C, under nitrogen protection with vigorous stirring. The as-formed solution was stirred at 30 °C at a constant pH value of 9(NH3·H2O was used to adjust pH value) for 1 h. The obtained precipitates were continuously mixed under constant heating at 50 °C for 30 min. After cooling to room temperature, the obtained magnetic nanoparticles (denoted as 6 nm Fe3O4) were several times washed with water and ethanol, ultrasonicated for 30 min, and then dried under vacuum at 60 °C overnight.
The typical procedure for the preparation of MWCNT–Fe3O4 was as follows: 3 g MWCNTs was dispersed into 150 mL of concentrated HNO3 (37 % by mass fraction), followed by oil bath heating at 110 °C for 5 h [8, 9]. The solution was diluted by deionized water until the pH value of the filtrate was around 7. Such treatment decorated –OH and –COOH groups onto the surface of MWCNT, which was confirmed by infrared spectrum analysis. After further rinsing and drying, 200 mg acid-purified nanotubes and 50 mg Fe3O4 nanoparticles were dispersed in 40 mL solution of deionized water/ethanol (1:1, volume ratio). The mixture was ultrasonicated for 1 h and then stirred at room temperature for 96 h. The solution was separated from the residue using 200 nm filter membrane, followed by vacuum drying at 50 °C for 16 h. A simplified scheme of MWCNT–Fe3O4 is illustrated in Fig. 1.
Results and discussion
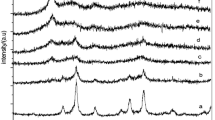
XRD analysis
XRD powder patterns of MWCNT–COOH and MWCN–Fe3O4 hybrid are shown in Fig. 2a and b respectively. The diffraction peaks at 2theta of 26,088 and 43,088 are attributed to the graphite structure (002) and (100) planes of the MWCNTs [10] in Fig. 2a, b showing that the hybrid is composed of two phases: cubic Fe3O4 and MWCNTs. No obvious peaks from other phases were observed [11]. The main peaks of Fe3O4 in the XRD pattern are broadened, indicating the smaller crystallite size of Fe3O4 NPs [10]. The mean size of the crystallites of Fe3O4 in the MWCNT–Fe3O4 (assuming spherical morphology) was estimated as 9 ± 3 nm from the diffraction pattern by X-ray line profile fitting [11].
FTIR analysis
The FTIR spectra of the samples are shown in Fig. 3. The band observed near 1,580 cm−1 in all samples shows the presence of the cylinder like carbon structure (rolled graphene sheet). According to Jishi et al. [12], several infrared active modes may have a wavenumber near 1,580 cm−1, while the wavenumbers depend on the geometry of the CNT. Besides, infrared wavenumbers are dependent on the nanotube diameter. The broadness of the band observed at 1,580 cm−1 for SWCNT samples can be explained by the polydispersity in the geometry of nanotubes. The stretching C=O vibration of the carboxyl (COOH) group is visible in the spectra of the modified carbon nanotubes. It was also observed at 1,713 cm−1 for MWCNT–COOH, and at 1,724 cm−1 for MWCNT–Fe3O4. Compared to MWCNT–COOH, this band reduced in the spectrum of MWCNT–Fe3O4. This reduction was ascribed to the linkage between magnetite particles and nanotubes, which was formed by a reaction of carboxyl groups with the surface of magnetite particles [13]. In the spectra of MWCNT–Fe3O4, the broadband around ca. 585 cm−1 showed the presence of iron oxide, primarily magnetite [13].
TEM analysis
As shown in Fig. 4, the morphologies of the samples were observed by TEM.
Figure 4 depicts an entangled network of acidified MWCNTs, which are attached to clusters of iron oxides. The iron oxide nanoparticle aggregates were composed of even smaller subunits of about 10 nm [14], implying that large particles may be formed via precipitation followed by a step-like aggregation process. In Fig. 4, MWCNTs are covered with the carboxylic acid (COOH) component in which iron oxide nanoparticles were uniformly dispersed without obvious aggregation. The carboxylic acid component grafted on the surface of the MWCNT was not destroyed in the process of loading iron oxides. Carboxylic acid can form complexes with metal ions because of their high number of coordinating functional groups [15]. This Figure shows representative TEM images of the MWCNT–Fe3O4 nanocomposites.
SEM analysis
Scanning electron microscopy shown in Fig. 5 is the typical morphology of the MWCNT–COOH (Fig. 5a), and MWCNT–Fe3O4 (Fig. 5b, c). SEM image (Fig. 5b, c) of the composites depicts an entangled network of oxidized MWCNTs (MWCNT–COOH) with clusters of iron oxides attached to them. The Surface area of the prepared composite was measured using BET method. The specific surface area of MWCNT–Fe3O4 composite was 92 m2/g. Under the reaction conditions employed, four iron oxides are commonly formed. These are Fe3O4 (magnetite), γ-Fe2O3 (maghemite), α-Fe2O3 (hematite) and α-FeO(OH) (goethite). Among them, two magnetite and maghemite are magnetic [16]. Most of the nanoparticles formed showed a tendency to congregate. Similar results were reported by Fan and Li [13].
TGA
TGA was used to characterize the iron oxide content in the as-obtained composites. For the iron oxide sample (Fig. 6c), the weight increase at the broad temperature is attributable to the oxidation of the Fe3O4 into Fe2O3. The TGA curve of MWCNTs (Fig. 6a) shows a small mass loss at lower temperature because of the removal of absorbed water and the functional groups. An obvious weight loss in the temperature range of 635–730 °C is caused by the oxidization of the nanotubes [17]. Compared with the pristine MWCNTs, the MWCNT–Fe3O4 composites lost their weight at a lower temperature range of 490–635 °C (Fig. 6b), leaving iron oxide residue weight of around 26.65 % for 6 nm Fe3O4/MWCNT a. This is attributed to the catalytic role of metal oxide nanoparticles in the oxidation of carbon materials [18].
VSM analysis
The magnetic property of the MWCNT–Fe3O4 nanocomposite was investigated by VSM. Figure 7 shows the magnetization curves measured at 25 °C for the sample fabricated at 200 °C for 12 h. Magnetization increased with an increase in the magnetic field. MWCNT–COOH possessed good magnetic properties, although the saturation magnetization was slightly lower than that of MWCNT–Fe3O4. Both compounds exhibited an extremely small hysteresis loop and low coercivity, as typically characteristic of superparamagnetic particles [19]. It is worth mentioning, this analysis shows that the saturation magnetization of the magnetic MWCNT composites was different and is significantly higher at functionalized samples.
Conclusions
A simple, effective and reproducible method has been carried out for synthesizing MWCNT–Fe3O4 magnetic nanocomposites. Magnetic measurements proved that MWCNT–Fe3O4 composite had superparamagnetic characteristics at room temperature. This study would help to develop a new composite material with excellent properties employed in large-scale fabrication of magnetic CNT hybrid materials.
References
Iijima, S.: Helical microtubules of graphitic carbon. Nature 354(6348), 56–58 (1991)
Ouyang, M., Huang, J.L., Lieber, C.M.: One-dimensional energy dispersion of single-walled carbon nanotubes by resonant electron scattering. Phys. Rev. Lett. 88(6), 066804 (2002)
Thostenson, E.T., Ren, Z.F., Chou, T.W.: Advances in the science and technology of carbon nanotubes and their composites: a review. Comp. Scien. Tech. 61(13), 1899–1912 (2001)
Bahr, J.L., Mickelson, E.T., Bronikowski, M.J., Smalley, R.E., Tour, J.M.: Dissolution of small diameter single-wall carbon nanotubes in organic solvents? Chem. Commun. 2, 193–194 (2001)
Singh, S., Barick, K.C., Bahadur, D.: Functional oxide nanomaterials and nanocomposites for the removal of heavy metals and dyes. Environment 4, 5 (2013)
Giersig, M., Hilgendorff, M.: Magnetic nanoparticle superstructures. Eur. J. Inorg. Chem. 18, 3571–3583 (2005)
Kanagesan, S., Hashim, M., Tamilselvan, S., Alitheen, N. B., Ismail, I., Hajalilou, A., Ahsanul, K.: Synthesis, characterization, and cytotoxicity of iron oxide nanoparticles. Adv. Mater. Sci. Eng. 2013 (2013)
Chen, W., Pan, X., Bao, X.: Tuning of redox properties of iron and iron oxides via encapsulation within carbon nanotubes. J. Am. Chem. Soc. 129(23), 7421–7426 (2007)
Fan, X.J., Li, X.: Preparation and magnetic properties of multiwalled carbon nanotubes decorated Fe3O4 nanoparticles. Carbon 50(10), 3962 (2012)
He, F., Fan, J., Ma, D., Zhang, L., Leung, C., Chan, H.L.: The attachment of Fe3O4 nanoparticles to graphene oxide by covalent bonding. Carbon 48(11), 3139–3144 (2010)
Gupta, V.K., Agarwal, S., Saleh, T.A.: Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res. 45(6), 2207–2212 (2011)
Jishi, R.A., Venkataraman, L., Dresselhaus, M.S., Dresselhaus, G.: Phonon modes in carbon nanotubules. Chem. Phys. Lett. 209(1), 77–82 (1993)
Fan, X.J., Li, X.: Preparation and magnetic property of multiwalled carbon nanotubes decorated by Fe3O4 nanoparticles. New Carbon Mater. 27(2), 111–116 (2012)
Chang, P.R., Zheng, P., Liu, B., Anderson, D.P., Yu, J., Ma, X.: Characterization of magnetic soluble starch-functionalized carbon nanotubes and its application for the adsorption of the dyes. J. Hazard. Mater. 186(2), 2144–2150 (2011)
Yao, Z., Braidy, N., Botton, G.A., Adronov, A.: Polymerization from the surface of single-walled carbon nanotubes-preparation and characterization of nanocomposites. J. Am. Chem. Soc. 125(51), 16015–16024 (2003)
Perales Perez, O., Umetsu, Y., Sasaki, H.: Precipitation and densification of magnetic iron compounds from aqueous solutions at room temperature. Hydrometallurgy 50(3), 223–242 (1998)
Zhang, M., Yudasaka, M., Bandow, S., Iijima, S.: Thermogravimetric analysis for the array of C60 molecules formed in single-wall carbon nanotube. Chem. Phys. Lett. 369(5), 680–683 (2003)
Li, J., Tang, S., Lu, L., Zeng, H.C.: Preparation of nanocomposites of metals, metal oxides, and carbon nanotubes via self-assembly. J. Am. Chem. Soc. 129(30), 9401–9409 (2007)
Liu, S., Zhang, L., Zhou, J., Xiang, J., Sun, J., Guan, J.: Fiberlike Fe2O3 macroporous nanomaterials fabricated by calcinating regenerate cellulose composite fibers. Chem. Mater. 20(11), 3623–3628 (2008)
Acknowledgments
HS, RS and MK would like to thank Islamic Azad University for all supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sadegh, H., Shahryari-ghoshekandi, R. & Kazemi, M. Study in synthesis and characterization of carbon nanotubes decorated by magnetic iron oxide nanoparticles. Int Nano Lett 4, 129–135 (2014). https://doi.org/10.1007/s40089-014-0128-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-014-0128-1