Abstract
Background
Cellulase is one of the enzymes commonly used in several agricultural, industrial and sewage sludge treatment processes. The present study aimed to investigate the potential use sludge generated from sewage treatment plants as a production medium for cellulase by B. megaterium strain that was isolated from a sewage treatment plant. The production of cellulase in the sludge medium was compared to different cellulosic materials: cotton, filter paper, bagasse and sawdust as well as to galactose, fructose, lactose, maltose, mannitol, mannose, ribose, sucrose and xylose. The production of cellulase was conducted at optimum conditions (0.4 mL of the bacterial inoculum, 45 °C, 72 h, pH 6.5 and citrate phosphate buffer) that were determined in this study.
Results
The sludge medium has induced the cellulase production by B. megaterium strain compared to cotton, filter paper, bagasse and sawdust. However, B. megaterium produced high cellulase in the presence of carbohydrate compounds as carbon source. More cellulase was produced in the sludge medium containing low concentrations of Ni2+, Zn2+ and Cu2+ ions.
Discussion
The ability of B. megaterium strain to produce cellulase in the sewage sludge medium was due to that the strain has acclimatized to resist heavy metals and produce the enzyme genetically. Moreover, B. megaterium has an important environmental role for reuse of sewage sludge as production medium for cellulase that could be used in many of applications, including production of animal feed, formulation of detergents, juice clarification, paper industry and wine production.
Similar content being viewed by others
Introduction
Cellulose is the most abundant renewable natural biological resource produced in the biosphere (about 100 billion dry tons per years) (Zhang et al. 2006). Municipal solid wastes contain 40–50 % cellulose, 12 % hemi-cellulose and 10–15 % lignin by dry weight (Wang et al. 1994). Several anaerobic bacteria have the ability to degrade cellulose in anaerobic digestion (Chynoweth and Pratap 1996). The degradation of cellulose by cellulase(s) enzymes produced by numerous microorganisms is very important in several agricultural and waste treatment processes (Hamer 2003; Angenent et al. 2004; Schloss et al. 2005). The aerobic microorganisms usually secrete copious amounts of free cellulase, which acts synergistically to degrade cellulose (Blouzard et al. 2007; Mingardon et al. 2007).
The widely accepted mechanism for enzymatic cellulose hydrolysis involves synergistic actions by endo-glucanase or CMCase (EC 3.2.1.4), exoglucanase or cellobiohyrolase (EC 3.1.1.91) and β-glucosidase (EC 3.2.1.21) (Lynd et al. 2002; Zhang and Lynd 2004; Sadhu et al. 2013). Endoglucanase hydrolyzes accessible intra-molecular β-1-4 glucosidic bonds of cellulose chain ends. Exoglucanases processively cleave cellulose soluble cellobiose or glucose and β-glucosidase hydrolyzes cellobiose to glucose (Krishna 1999; Zhang et al. 2006).
Applications of cellulase include production of animal feed, formulation of detergents, juice clarification, paper industry and wine production. Cellulase contributes to 8 % of the worldwide industrial enzyme demands and the demand is expected to increase by 100 % in future (Costa et al. 2008). However, production of cellulase(s) by different microorganisms was found to be affected by many factors, e.g. inocula size, incubation temperature, incubation period, pH value, buffers, carbon and nitrogen sources (Mawadza and Zvauya 1996; Camassola et al. 2004; Silva et al. 2005; Immanuel et al. 2006).
The type and concentration of carbohydrate are critical for maximal cellulase production in the production medium. Krishna (1999) has suggested that the glucose has enhanced cellulase synthesis to a significant level. However, addition of cellulose, lactose or glucose at concentration above 1 % level led to a significant reduction in enzyme synthesis. Alam et al. (2004) have studied the production of extracellular cellulase by S. omiyaensis under different carbon source availability. Four carbon sources have been investigated: carboxyl methyl cellulose (CMC), avicel, rice bran and sawdust at the rate of 1.2 %. They have revealed that the highest CMCase enzyme production was recorded when CMC was used as a carbon source and the lowest CMCase production when sawdust and rice bran were used as carbon sources.
Emtiazi et al. (2007) have revealed that Paenibacillus strain produced high CMCase when CMC was used as only carbon sources. Karim et al. (2014) have revealed that B. licheniformis produced a significant amount of cellulase when wheat bran and orange peel were used as a sole carbon source. However, the production of CMCase in the sludge medium has not been studied before. Therefore, the current work aimed to investigate the recycling of sludge as production medium of CMCase by Bacillus megaterium strain in comparison to cellulosic materials such as cotton, filter paper, bagasse and sawdust as well as to different carbon sources (galactose, fructose, lactose, maltose, mannitol, mannose, ribose, sucrose and xylose). The effect of different concentrations of nickel ions was also tested to investigate the potential of bacterial strain to produce the enzyme in different types of sludge contaminated with heavy metals.
Materials and methods
Collection of sewage sludge samples
Twelve sewage sludge samples were collected weekly from four STPs (referred to as ISTP, TSTP, ASTP and SSTP) in Yemen. TSTP was located in Taiz and treat Industrial waste generated from Industrial and Commercial Company. ISTP, ASTP and SSTP were located in Ibb, Aden and Sana’a, respectively. The sewage flows to these plants comprise residential, commercial and industrial. TSTP and ASTP are oxidation ponds. The treatment of sewage in the ISTP and SSTP is based on primary and secondary processes. The sludge samples were collected from each STP in sterile paper bags. The samples were transported to the laboratory in a cooler box and the microbiological analysis was carried out within 2 h of collection.
Determination of heavy metals (Cu2+, Ni2+ and Zn2+) concentrations
Heavy metals were extracted from dewatered sludge samples by nitric acid digestion method (APHA 1998). The heavy metal concentrations (Zn2+, Cu2+ and Ni2+) in the digested samples were analysed and determined by atomic absorption. An atomic absorption spectrophotometer (AAS) was used for this purpose.
Screening of bacterial isolates for resistance to nickel ions and production of cellulase
Hundred and twenty-seven (127) bacterial isolates were isolated from sludge samples collected from four sewage treatment plants in Yemen. These bacterial isolates were purified according to APHA, 9225B (1999). The screening for the bacterial isolates resistant to nickel ions was conducted according to Hernández et al. (1998) and Abdel-Monem et al. (2010). The bacterial isolates were sub-cultured in BHI agar medium containing 15 and 10 mM Ni2+ for 24–48 h at 35 °C. The plates were exposed to sulphhydryl gas (resulted from the reaction of 2 g of sodium sulphide with 10 mL of concentrated HCl in a sealed container, the reagent was prepared before each experiment) in a sealed container for the formation of the metal sulphide. Plates were carefully screened to detect any change in the region surrounding or inside the bacterial colonies.
The bacterial isolates were screened for the growth on CMC–Yeast Extract (CYE) agar medium containing (g L−1): NaNO3, 2; KCl, 0.5; MgSO4, 0.5; K2HPO4 (buffer), 1.0, yeast extract (growth factor), 1 and CMC as carbon source, 10; pH 7.0 ± 0.2. After incubation at 37 °C for 48 h, the isolates that exhibited good growth were recorded. To detect production of cellulase, the surface of the plates was floated with iodine ZnCl2 solution (3 % ZnCl2 was added to gram’s iodine solution). Diameters of blue zones were measured. The bacterial isolates that exhibited positive results for the production of cellulase were grown on CMC–Yeast Extract (CYE) broth medium for 5 days at 37 °C. Cellulase production was determined by CMC cup-plate clearing zone (CCZ) assay.
In this assay, 2 % (w/v) CMC was dissolved in citrate buffers (pH 7) and supplemented with 1.5 % agar for solidification. After sterilization, equal amounts of assay medium (20 mL) were poured in sterilized petri dishes (12 cm in diameter). Cups (10 mm in diameter) were made in each plate using a sterile cork borer. Equal amounts of the enzyme solution (cultural supernatant) were put into each cup. Plates with cups containing enzyme solutions were incubated at 37 °C for 24 h, then the surface of the plates was floated with iodine ZnCl2 solution. Diameters of blue zones were measured. The mean values of three readings were calculated.
Identification of the most potent bacterial isolates
The bacterial isolates that exhibited the ability in the accumulation of Ni2+ ions were identified based on morphological, culture and biochemical tests according to Noel (1984), Sneath et al. (1986), Garrity et al. (2002) and Brenner et al. (2004).
Cellulase production under catabolite repression
The purpose of this experiment was to investigate the ability of bacterial strains to produce cellulase enzyme as inducible or genetically. The medium used was as mentioned previously with glucose (1 % w/v) equivalent to CMC as the only carbon source. After 48 h incubation at 37 °C, the cellulase enzyme assay was done by C.C.Z assay as mentioned previously.
Factors affecting CMCase production by bacterial isolate No. 1295S
The bacterial isolate No. 1295S, which secretes the highest yield of cellulase under catabolic repression, was selected for studying different factors affecting CMCase production: inocula size, temperatures, incubation periods, pH values, buffers and nitrogen sources.
Inoculum preparation
The Bacterial isolate No. 1295S was maintained as stock culture on Brain Heart Infusion agar (BHIA). The bacterial strain was grown at 37 °C for 24 h and then stored at 4 °C for regular sub-culturing. The bacterium inoculum was prepared using BHI broth in 250 mL conical flask. The inoculum was kept in shaker (200 rpm) at 37 °C for 14 h before it was used for the CMCase production.
Production medium
Carboxyl methyl cellulose yeast extract (CYE) liquid medium was used for studying factors affecting the CMCase production. The constituents of 50 mL of this medium were dispensed in flasks of 250 mL capacity. The pH was adjusted at 6.5 and autoclaved at 121 °C for 15 min.
Biomass yield
The bacterial growth was determined by dry weight method. After collection of supernatant, the biomass residue was dried at 80 °C for 24 h and the yield was expressed as mg g−1 of substrate.
β-1,4 Endoglucanase (CMCase activity) determination
β-1,4 endoglucanase (CMCase activity) (EC 3.2.1.4) was determined by measuring the amount of reducing sugars released in the reaction mixtures containing 1.7 mL of 0.1 M acetate buffer (pH 5.5), 0.8 mL of 2 % (w/v) CMC (sigma) solution and 0.5 mL of the culture supernatant. The mixture was maintained at 50 °C for 50 min. The reducing sugars in the supernatant were assayed by 3,5-dinitrosalicylic acid (DNSA) methods (Miller 1959). Glucose was used as standard. Measurements were made in a spectrophotometer (Win. Aspect T 20, 031-2004, Germany) at 540 nm wavelength in the presence of the blank. The reaction mixtures containing heat-inactivated post-culture liquids (boiled for 5 min) were used as blanks. The cellulolytic enzyme activities were expressed in units defined as the quantity of enzyme required to produce 1 µmol/h of glucose, under the conditions of the assay.
Effect of different inocula sizes
The effect of different inocula sizes on the production of cellulase enzyme was investigated at 0.1, 0.2, 0.3, 0.4, 0.6, 0.8, 1, 1.5, 2 and 2.5 mL of bacterial inoculum (~2.7 × 109 CFU mL−1). After the inoculation, the production medium was incubated at 37 °C and pH 6.5 for 4 days. Detection of CMCase production was performed by the determination of reducing sugar using colorimetric technique as previously mentioned.
Effect of temperature
For this purpose, the production medium was dispensed into flasks of 250 mL capacity; each flask containing 50 mL of liquid medium was adjusted at pH 6.5. The flasks were autoclaved at 121 °C for 15 min. The sterile medium was inoculated with 0.4 mL (~2.7 × 109 CFU mL−1) of a standardized bacterial inoculum, and incubated at the following temperatures 20, 30, 37, 45 and 60 °C. At the end of the incubation period of 4 days, the CMCase produced was performed as described previously.
Effect of different incubation periods
The production medium was prepared as mentioned above where bacterial isolate no. 1295S was incubated for 1, 2, 3, 4, 5, 6 and 7 days at 45 °C and pH 6.5. At the end of each incubation period, the production of CMCase was determined using the colorimetric technique.
Effect of pH values
The pH values of the production medium were adjusted at 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5 and 8 by the careful addition of drop of (0.1 N) HCl and (0.2 N) NaOH using pH meter (IA 31-1114 WTW Germany). The media were dispensed in flasks of 250 mL capacity each containing 50 mL and then autoclaved at 121 °C for 15 min, then inoculated with 0.4 mL of a standardized bacterial inoculation. The inoculated media were incubated at 45 °C for 72 h.
Effect of different buffers applied at various pH ranges
CYE liquid medium was used for detecting the suitable buffer for CMCase production. The following buffers with their pH ranges were used for such a purpose, citrate buffer with pH range from 5.6 to 6.2, citrate phosphate buffer with pH range from 6 to 7 and phosphate buffer with pH range from 6 to 8. All these buffers with their different pH were prepared according to Collee et al. (1989). The constituents of the production medium were dissolved in the buffer solution and poured into the flask contained 50 mL of the buffered medium at the particular pH. The media were inoculated with 0.4 mL of a standardized bacterial inoculation. Then, the medium was incubated at 45 °C for 72 h. Extraction of the crude enzyme was carried out as mentioned above at the end of the incubation period and determined using colorimetric technique.
Effect of different cellulosic materials supplied at different concentration
This experiment was designed to test the effect of different cellulosic materials on the CMCase production. DCY liquid production medium was used without CMC. Substrates were singly supplied to production medium. These substrates were CMC, cellulose powder, cotton, filter paper, sawdust, bagasse and tobacco leaves. All substrates were supplied at concentrations 2, 4, 6, 8 and 10 mg mL−1 (w/v), respectively. pH value was adjusted to 6.5. The production medium was inoculated with bacterial inoculum and then incubated at 45 °C for 72 h.
Effect of different carbon sources applied at different concentrations
For this purpose, galactose, fructose, lactose, maltose, mannitol, mannose, ribose, sucrose and xylose were used at different concentrations (2, 4, 6, 8 and 10 mg mL−1) (w/v). They were supplied singly to the sterilized carbon-free production medium. The carbon sources used were sterilized by membrane filter. The production medium with CMC was used as control; other steps were carried out as mentioned previously.
Effect of different sludge media applied at different concentrations of sludge
The ability of B. megaterium strain to produce enzyme in the presence of sludge as only carbon and nitrogen source at different concentrations (2, 4, 6, 8 and 10 mg mL−1) (w/v) was studied. The production medium was inoculated with 0.4 mL of a standardized bacterial inoculum. At the end of the incubation period 72 h, the CMCase produced was detected using colorimetric technique.
Effect of Ni2+ ion concentrations
To study the effect of Ni2+ ion concentrations on CMCase production, the (CYE) liquid medium was prepared with concentrations 117.2, 234.4, 351.6, 468.8, 586, 703.2 and 820.4 µg Ni2+ mL−1. The medium was inoculated with bacterial strain and then incubated at 45 °C for 72 h. At the end of the incubation period, CMCase concentration was determined as described previously.
Data analysis
All analyses were conducted in triplicate and values were reported as means with standard deviations. Data were subjected to one-way analysis of variance (ANOVA) in the general linear model using the SPSS 11.5 statistical package. The statistical package (EASE, M-STAT) was used to perform the analyses of least significance difference (LSD). ANOVA was used to determine the significance (p < 0.05) of the differences between results.
Results and discussion
Selection of the most potent bacterial strains
One-hundred and twenty-seven bacterial isolates were obtained from sewage sludge samples collected from ISTP, TSTP, ASTP and SSTP at republic of Yemen. These isolates were purified and screened for nickel resistance at concentrations of 15 and 10 mM of nickel ions, where the highest value of nickel ions was 15 mM in sludge sample at AWTP (Table 1).
Among the 127 bacterial isolates, three bacterial isolates (586S, 1295S and 222W) exhibited good growth in the presence of nickel concentrations of 15 mM (876 µg Ni2+ mL−1) and two bacterial isolates (117S and 120S) showed high growth at 10 mM (584 µg Ni 2+ mL−1).
Bacterial isolates forming a dark colour around or inside the colony (possible reduction and precipitation of the metal) were considered to be possible nickel bio-sorbents. Five bacterial isolates producing dark colour colonies grown in the presence of nickel were selected for further studies. Neither clear halos nor dark colours were observed around the colony when the clinical strains (E. coli, control) were grown in the presence of nickel ion concentrations. The bacterial isolate Nos. 586S, 1295S and 222W were characterized by a higher nickel tolerance than the bacterial isolate Nos. 117S and 120S.
Survey for nickel resistance among the obtained bacterial isolates was chosen because nickel is among the most toxic heavy metals and could be found in different industries (Kaewchai and Prasertsan 2002). Ni2+ is one of the most frequently encountered heavy metals in sewage streams (Padmavathy 2008). Ni2+ is a trace element and plays a role as cofactors for some of the bacterial enzymes (Nies 1999; Dosanjh and Michel 2006).
Only few bacterial strains were described to grow in the presence of higher concentration than 10 mM of Ni2+. Hernández et al. (1998) found that from 52 bacterial strains isolated from contaminated soils of an oil refinery, only two strains of bacteria Escherichia hermannii and Enterobacter cloacae were capable of accumulating either nickel, vanadium or both metals at 10 mM. Leung et al. (2000) isolated nineteen metal resistant and non-resistant bacteria of Ni2+, Pb2+, Cu2+ and Zn2+ from the activated sludge. In the present work, among the 127 bacterial isolates obtained from sludge, five bacterial isolates exhibited resistance to 10 mM of Ni2+ ions. Al-Gheethi et al. (2014) reported that Bacillus sp., Pseudomonas sp., Chryseomonas sp., and Burkholderia sp. isolated from sewage effluent have the ability to tolerate 6 mM of Ni2+ ions.
The results of screening for cellulase production showed that 69 (almost 54.33 %) from 127 isolates exhibited high growth, 59 from 69 bacterial isolates produced clearing zones at varying degree and considered as positive for cellulase(s) production. It has been reported that the clear zone methodology was developed to isolate polymer-degrading strains from mixtures of microorganisms typically found in environments such as compost or soil. Microorganisms capable of degrading a polymeric material are determined by looking for zones of clearing around microbial colonies on agar media that are opaque due to the presence of powdered polymer (Pettigrew and Johanson 1996; Ten et al. 2004). This screening process was performed in this work to choose the bacterial isolates, which have the potential to grow in the CYM medium containing CMC as carbon source.
The second screening was carried out by growing the bacterial isolates, which have positive results in the previous screening in the liquid medium for confirmation of the cellulase production using C.C.Z method. In this screening, the diameters of the clearing zones surrounding the wells on the plate screening medium ranged from 11 ± 0.4 to 77.5 ± 7.4 mm. This screening step was found to give reliable indication of exhibited cellulolytic activities. However, the enhancement of cellulase production was not linked with the amount of bacterial growth. The size of clearing zone diameter of each isolate is depicted in Fig. 1. Results showed that among the 59 bacterial isolates which exhibited clear zone around the colony in the last screening, 42 isolates (71.2 %) exhibited potential to produce cellulase enzyme as determined by C.C.Z technique. Among the forty-two cellulolytic bacterial isolates, five bacterial isolates (586S, 1295S, 117S, 120S and 222W) showed nickel tolerance and selected for further studies as will be described below.
Identification of the most potent bacterial isolates
The bacterial isolates Nos. 586S, 1295S, 222W, 117S and 120S which showed high nickel tolerance were identified as Sporosarcina pasteurii 586S, Bacillus megaterium 1295S, Staphylococcus xylosus 222W, Bacillus subtilis 117S and Pseudomonas cepacia 120S (Table 2).
Production of cellulase under catabolic repression
The considerations in the enzymatic treatment of sludge are the presence of substrates such as glucose, which may inhibit the production of enzymes by the added bacterial strains. The ability of bacterial strains to produce detectable amounts of CMCase under catabolic repression (genetically) was screened in the presence of glucose (1 % w/v) equivalent to CMC as sole carbon source. As shown in Fig. 2, it could be observed that B. megaterium and B. cepacia could still synthesize cellulase with varying degrees. These results were in agreement with Allcock and Woods (1981) who stated that the CMCase in Clostridium acetobutylicum induced by molasses and it was not repressed by glucose. S. pasteurii, B. subtilis and S. xylosus appeared to lose the ability to produce the enzyme in the presence of glucose. Bakare et al. (2005) demonstrated that cellulase activity by P. fluorescens increased when cellulose material was added to the culture medium than when glucose was used as sole carbon source. In this work, B. megaterium 1295S produced CMCase in CYE2 medium (CMC–yeast extract agar medium containing glucose as carbon source) more than that in CYE1 (CMC–yeast extract agar medium containing CMC as carbon source) medium whereas B. cepacia 120S produced highest amounts of enzyme on CYE1 medium. B. megaterium 1295S has produced cellulase on CYE2 twofold than on CYE1 and indicating the ability to produce cellulase genetically. Conversely, B. cepacia 120S and S. xylosus 222W have described as inducible enzyme production.
Production of cellulase(s) enzymes under catabolite repression by five bacterial strains. Each point is the average of three determinations. CYE1 CMC–Yeast Extract agar medium containing carboxyl methyl cellulose (CMC) as carbon source. CYE2 CMC–Yeast Extract agar medium containing glucose as carbon source
Al-Gheethi and Norli (2014) studied the production of β-lactamase by bacteria isolated from sewage effluents in the presence or absence of cephalexin antibiotic as inducible substrate. They found that B. stearothermophilus and Burkholderia cepacia could still synthesize β-lactamase at varying conditions. Chryseomonas luteola has lost the ability to produce the enzyme in the absence of cephalexin (as inducibly). B. subtilis has produced β-lactamase in antibiotic-free and induced medium and considered as producing β-lactamase genetically. In the current study, the bacterial species, which produced highest cellulase in the presence of glucose, were regarded as catabolite repression resistant and have constitutively CMCase production.
Factors affecting production of CMCase by B. megaterium strain
B. megaterium strain was selected for studying the factors affecting CMCase production. The selection of B. megaterium strain was based on that the strain has produced cellulase under catabolite repression and has the ability to grow at 15 mM Ni2+ ions. The factors investigated were inocula size, incubation temperatures, incubation periods, pH values, different buffers applied at various pH ranges. In these experiments, the amount of CMCase was determined by measuring reducing sugars using the 3,5-dinitrosalicylic acid (DNSA) methods as described in “Materials and methods” (Miller 1959). The DNSA methods were used because the determination of differences in enzyme activity levels less than twofold is difficult using C.C.Z techniques (Zhang et al. 2006). The DNSA method is one of the most common assays for measuring reducing sugars for cellulase activity assays because of their relatively high sugar detection range (Coward-Kelly et al. 2003; Kongruang, et al. 2004; Zhang and Lynd 2005).
The maximum amount of CMCase production (16.8 U mL−1) was noted with 0.4 mL of the bacterial suspension (Table 3). The optimum inocula size for heavy growth was 0.1 mL. Krishna (1999) studied the effect of inoculum size (5–40 %) on production of exoglucanase and endoglucanase by B. subtilis and revealed that 15 % (v/w) of inoculum size was optimal for the production of both enzymes. Immanuel et al. (2006) reported that one millilitre inoculum of Bacillus spp., Cellulomonas spp. and Micrococcus spp. was optimal to produce cellulase enzyme.
The high production of CMCase (35.1 U mL−1) and biomass yield (710 mg g−1) by B. megaterium strain was recorded at temperature 45 °C (Table 4). Alam et al. (2004) observed similar results; the maximum production of CMCase by Streptomyces omiyaensis occurred at 45 °C, while the maximum growth was recorded at the range from 35 to 40 °C. The range between 35 and 60 °C was reported as optimal temperature of growth and production of cellulase by Bacillus sp. (Chan and Au 1987; Krishna and Varma 1990; Mawadza and Zvauya 1996; Ito 1997; Krishna 1999; Immanuel et al. 2006; Sadhu et al. 2013; Sethi et al. 2013).
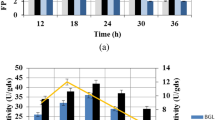
The overproduction of CMCase (32.5 U mL−1) was obtained after 72 h of incubation period (Table 5). The amount of growth increased simultaneously with time; the maximum yield of cell growth of B. megaterium was noted after 4 days. The enzyme production was unstable and decreased rapidly after 72 h. Reduction in CMCase yield was accompanied by an accumulation of reducing sugars in the medium and compatible with increasing amount of growth at 7 days. Krishna (1999) found similar trend in cellulase production using B. subtilis and indicated that the decrease in activity after 72 h might be due to denaturation of the enzyme, resulting from variations in pH during incubation. Maximal production in Bacillus strains has achieved after 2–3 days (Kawai et al. 1988; Ito et al. 1989). The production of CMCase by B. megaterium in a continuous-operating reactor may keep the bacterial growth at the highest stage of enzyme production. However, the current study focused on the batch scale of bacteria and continuous-operating reactor will be conducted in the possible future work.
The maximum CMCase production was noted at pH value of 6.5 (Table 6). Further increase in pH level to 8 resulted in considerable decrease in enzyme production. At pH 6, the B. megaterium showed heavy growth, but at pH 4 showed little growth and at pH 8 showed moderate growth, respectively. The optimum pH value of cellulolytic organisms varied from acidic condition such as Trichoderma reesei pH 2.8–pH 3.5 (Sternberg and Mandels 1979) to alkaline conditions such as Bacillus sp. pH 9–12 (Hakamada et al. 1997). Alam et al. (2004) revealed that S. omiyaensis showed heavy growth and high cellulase activity at pH 6.5. The optimal condition of cellulase production by Bacillus sp. was recorded at pH range from 5 to 7.5 (Dhillon et al. 1985; Araujo and Ward 1990; Mawadza and Zvauya 1996; Krishna 1999; Immanuel et al. 2006; Karim et al. 2014).
The effect of buffers on production of CMCase is illustrated in Table 7. Highest CMCase production was attained when the initial pH of the medium was adjusted to pH 6.2 and 6.4 with phosphate buffers, where 38.5 U mL−1 was produced. The production of CMCase in the medium with citrate phosphate buffers was 38.6 and 38.4 U mL−1 at pH 6.4 and 6.6. The minimum production of CMCase was noted with the citrate buffer 33.7 U mL−1 at pH 6.2 and non-buffered production medium 34.2 U mL−1 at pH 6.5. The optimum buffers of heavy growth were observed as citrate phosphate 1260 mg g−1 at pH 6.2 and phosphate buffers 1400 mg g−1 at pH 7.4 were used in culture medium, respectively. These results indicated that phosphate buffer is an important buffer for cellulase production by B. megaterium. Stutzenberger (1971) showed that maximum cellulase production by Thermomonospora curvata was attained when the initial pH of the medium was adjusted to pH 8.0 with phosphate buffer. However, Duff et al. (1985) stated that sodium citrate buffer improved the enzyme production in production medium by up to 40 %. Camassola et al. (2004) reported that production of cellulase enzyme by Penicillium echinulatum with citrate buffer was slightly higher than that in acetate buffer of the same pH.
Effect of different cellulosic material and carbon source on the production of CMCase
The effect of different cellulosic material was studied to determine the optimum concentration of each substrate supplied for the production of CMCase. Five concentrations of each substrate were used: 2, 4, 6, 8 and 10 mg mL−1 (w/v). The results (Table 8) show that the maximum amount of CMCase production (52.7 U mL−1) was recorded at 8 mg mL−1 of CMC compared to 22.8 U mL−1 at 6 mg mL−1 of cellulose powder. The lowest cellulase production was noted at 2 mg mL−1 of cotton and 6 mg mL−1 of sawdust. The highest saccharification (10 %) was observed when CMC was used as carbon source followed by cellulose powder.
The effect of fructose, galactose, lactose, maltose, mannitol, mannose, ribose, sucrose and xylose on CMCase production is presented in Table 8. The results indicate that the maximum production of CMCase (51.4 U mL−1) was recorded when 10 mg mL−1 of mannose was used as carbon source followed by 8 mg mL−1 of ribose (47.4 U mL−1), 8 mg mL−1 of fructose (45.5 U mL−1), 8 mg mL−1 of xylose (44.8 U mL−1), 2 mg mL−1 of lactose (40.8 U mL−1) and 4 mg mL−1 of mannitol (19.3 U mL−1). The lowest production of CMCase (11.7 U mL−1) was recorded with 8 mg mL−1 of galactose, maltose and 6 mg mL−1 of sucrose. The highest saccharification (%) was observed with xylose and ribose, 9.7 and 9.5 %, respectively. The results obtained in the current study are similar to those reported by Alam et al. (2004) who found that the highest CMCase production was recorded when CMC was used as carbon source and the lowest CMCase production with sawdust as a carbon source. Paenibacillus produced (4 U mL−1) CMCase when it was grown on CMC as the only source of carbon (Emtiazi et al. 2007). Krishna (1999) suggested that addition of cellulose powder, lactose or glucose at concentration above 1 % level led to a significant reduction in enzyme synthesis by B. subtilis.
Recycling of sludge as production medium for CMCase
The production of CMCase by B. megaterium inoculated to sludge samples was investigated in the present study. The results are presented in Fig. 3. The maximum amount of CMCase production (14.3 U mL−1) was recorded in 6 mg mL−1 (w/v) of sludge from SSTP, followed by sludge collected from TWTP at concentration 4 mg mL−1, where 13.1 U mL−1 was produced. The lowest amount of CMCase production (1.4 U mL−1) was observed in sludge collected from ASTP and ISTP (1.6 U mL−1). The highest saccharification (7.5 %) was observed when 10 mg mL−1 (w/v) of sludge from SSTP was used as production medium, while the lowest saccharification (0.3 %) was noted with 8 mg mL−1 (w/v) of sludge from ASTP. The production of CMCase in sludge medium has not been reported before. However, Barros et al. (2013) used cassava wastewater as production medium for amylase, protease and lipase by B. subtilis strains and revealed that the bacteria produce detectable amounts of these enzymes in comparison to the synthetic liquid medium. Al-Gheethi and Norli (2014) investigated the production of β-lactamase in sewage effluents medium by B. subtilis, C. luteola and B. cepacia. They revealed that the bacterial strains produced β-lactamase at levels above 0.333 U mL−1.
Effect of different sludge media applied at different concentrations of sludge on saccharification and CMCase production by B. megaterium. a Sludge from Ibb sewage treatment plant, b sludge from Taiz sewage treatment plant, c sludge from Aden sewage treatment plant, d sludge from Sana’a sewage treatment plant
In comparison to the concentrations of heavy metals in sludge collected from the STPs, it can be noted that the concentration of Cu2+ and Zn2+ ions in the sludge from TSTP was more than that in the sludge from ASTP and ISTP. However, the production of CMCase and percentage of saccharification in sludge from TSTP were more than those in the sludge from ASTP and ISTP. These results could be explained based on the toxicity of Ni2+ ions in comparison to Cu2+ and Zn2+ at the high concentration. It has reported that Ni2+, Cu2+ and Zn2+ improve the enzymatic reaction at low concentration (Nies 1999). However, at high concentrations, Ni2+ becomes more toxic than Cu2+ and Zn2+. This is because the transport of Zn2+ and Cu2+ through bacterial cells membrane is by specific transport system (Fath and Kolter 1993; Fagan and Saier 1994), while Ni2+ is accumulating by the fast and unspecific system (Smith and Maguire 1995; Tao et al. 1995). Besides, the ability of bacteria to resist Cu2+ was reported to be more than Ni2+ at the same concentrations (Al-Gheethi et al. 2014). Lankinen et al. (2011) reported that the production of β-glucosidase and β-cellobiosidase by decomposing fungi in heavy nickel-contaminated soil (more than 20 mg kg−1) was less than that in the non-contaminated soil. In this study, however, the ability of B. megaterium to produce detectable amount of CMCase in sludge contaminated with Ni2+ ions is because B. megaterium was tolerant to 15 mM of nickel. Therefore, this bacterium would be used to produce cellulase from the sludge.
Effect of different nickel ion concentrations
The effect of different nickel ion concentrations was investigated to know the minimal Ni2+ ion concentrations at which B. megaterium strain produces maximum amount of CMCase. Ni 2+ ion concentrations of 117.2–468.8 µg Ni2+ mL−1 have provided a broad range for the growth of bacteria and stimulated CMCase production with optimal production at 468.8 µg Ni2+ mL−1. There was little detectable growth above 468.8 µg Ni2+ mL−1 or cellulase production below 351.6 µg Ni2+ mL−1 or above 586 µg Ni2+ mL−1. The maximum biomass yield and CMCase production (450 mg g−1 and 9.1 U mL−1 respectively) was obtained at 117.2 and 468.8 µg Ni2+ mL−1 of the nickel ions, respectively (Fig. 4).
Nickel has been demonstrated for many bacteria and plant species, where eight nickel-containing enzymes present in one or more of these species have been identified (Ragsdale 1998; Wattt and Ludden 1999). Nickel is identified as micronutrients at trace concentrations. On the other hand, it has been noted that the addition of Ni2+ ions at low concentrations enhanced biomass yield (Sujarittanonta and Sherrard 1981). Husain et al. (2013) revealed that the biomass of P. fluorescens increased with increasing Ni2+ ions in the broth medium from 250 to 1000 mg L−1; the maximum growth was recorded at 1000 mg L−1. However, the ability of Ni2+ ions to induce the CMCase production by B. megaterium strain has not been reported before.
In comparison to the production of CMCase in the sludge medium, the concentrations of nickel ions in the sludge from TSTP and SSTP were 156 and 26 mg kg−1, while the maximum concentrations of nickel added to the production medium were 820.4 µg L−1. However, the amount of CMCase produced in the sludge medium was more than that in the CYE medium. The increasing CMCase production in the sludge may be related to the sludge which is rich in nutrients and trace elements that induced the enzyme production more than CYE medium that is a synthetic medium. These findings are in consistent with those reported by Barros et al. (2013). They revealed that the production of amylase, protease and lipase by B. subtilis strains in cassava wastewater was more than that in the synthetic liquid medium. Another explanation of high production of CMCase in the sludge medium may be due to the nitrogen source. The sludge is rich with the amino acid and organic compounds, which represent as nitrogen source of bacteria, while in CYE the nitrogen source used was inorganic (sodium nitrate). Al-Gheethi (2008) revealed that the production of cellulase in the presence of peptone as nitrogen source was more when sodium nitrate was used as nitrogen source.
Conclusions
It can be concluded that B. megaterium strain isolated from the sludge possesses an important potential to produce CMCase in sewage sludge medium. The potential of bacterial strain to use sewage sludge as production medium would lead to biodegradation of cellulose in the sludge and, thus, can be used as a source of biofuel.
References
Abdel-Monem MO, Al-Zubeiry AH, Al-Gheethi AAS (2010) Biosorption of nickel by Pseudomonas cepacia 120S and Bacillus subtilis 117S. Water Sci Technol 61:2994–3007
Alam MZ, Manchur MA, Anwar MN (2004) Isolation, purification and characterization of cellulolytic enzyme produced by the isolate Streptomyces omiyaensis A2. Pak J Biotechnol Sci 7(10):1647–1653
Al-Gheethi AAS (2008) Bacteriological studies on sludge from some municipals wastewater treatment plants in Yemen. MSc Thesis, Department of Applied Microbiology, Faculty of Applied Science, Taiz University, Taiz, Yemen
Al-Gheethi AAS, Norli I (2014) Biodegradation of pharmaceutical wastes in treated sewage effluents by Bacillus subtilis 1556WTNC. Environ Process 1:459–489
Al-Gheethi AAS, Norli I, Lalung J, Megat-Azlan A, Nur-Farehah ZA, Ab. Kadir MO (2014) Biosorption of heavy metals and cephalexin from secondary effluents by tolerant bacteria. Clean Technol Environ Policy 16:137–148
Allcock ER, Woods DR (1981) Carboxymethyl cellulase and cellobiase production by Clostridium acetobutylicum in an industrial fermentation medium. Appl Environ Microbiol 41(2):539–541
Angenent LT, Karim K, Al-Dahhan MH, Wrenn BA, Domíguez-Espinosa R (2004) Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol 22:477–485
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
APHA (1999) Standard methods for the examination of water and wastewater, 9225B, 21st edn. American Public Health Association, Washington, DC
Araujo A, Ward OP (1990) Hemicellulases of Bacillus sp. preliminary comparative studies of production and properties of mammamases and galactanases. J Appl Bacteriol 68:1253–1261
Bakare MK, Adewale IO, Ajayi AO, Shonukan OO (2005) Purification and characterization of cellulase from the wild-type and two improved mutants of P. fluorescens. Afr J Biotechnol 4(9):898–904
Barros FFC, Simiqueli APR, de Andrade JC, Pastore GM (2013) Production of enzymes from agroindustrial wastes by biosurfactant-producing strains of Bacillus subtilis. Biotechnol Res Int 2013:1–9
Blouzard JC, Bourgeois C, Philip P, Valette O, Bélaïch A, Tardif C, Bélaïch JP, Pagès S (2007) Enzyme diversity of the cellulolytic system produced by Clostridium cellulolyticum explored by two-dimensional analysis: identification of seven genes encoding new dockerin-containing proteins. J Bacteriol 189(6):2300–2309
Brenner DJ, Krieg NT, Staley JT (2004) Bergeys manual of systematic bacteriology, The Proteobacteria, 2nd edn, vol 2, part A, B and C. Williams and Wilkins Awaverly Co, USA
Camassola M, De Bittencourt LR, Shenem NT, Andreaus J, Dillon AJP (2004) Characterization of cellulase complex of Penicillium echinulatum. Biocat Biotrans 22(5–6):391–396
Chan KY, Au KS (1987) Studies on cellulase production by Bacillus subtilis. Antonie Van Leeuwenhoek 53(2):125–136
Chynoweth DP, Pratap P (1996) Anaerobic digestion of municipal solid wastes. In: Palmisano AC, Barlaz MA (eds) Microbiology of solid waste. CRC Press Inc, Florida, pp 71–104
Collee JG, Duguid JP, Fraser AG, Marmion BP (1989) Practical medical microbiology, 13th edn. Longman-FE. Ltd, London
Costa RB, Silva MVA, Freitas FC, Leitao VSF, Lacerda PSB, Ferrara MA, Bon EPS (2008) Mercado e Perspectivas de Uso de Enzimas Industriais e Especiais no Brasil. In: Bon EPS, Ferrara MA, Corvo ML, Vermelho AB, Paiva CLA, Alencastro RB, Coelho RRR (eds) Enzimas em Biotecnologia, Producao, Aplicac oes e Mercados, 1st edn. Rio de Janeiro, Intercie^ncia, p 463–488
Coward-Kelly G, Alello-Mazzari C, Kim S, Granda C, Holtzapple M (2003) Suggested improvement to the standard filter paper assay used to measure cellulase activity. Biotechnol Bioeng 82:745–749
Dhillon N, Chhibber S, Saxena M, Pajni S, Vadehra DV (1985) A constitutive endoglucanase (CMCase) from Bacillus licheniformis. Biotechnol Lett 7(9):695–697
Dosanjh NS, Michel SL (2006) Microbial nickel metal-loregulation NikRs for nickel ions. Curr Opinion Chem Biol 10(2):123–130
Duff SJB, Cooper DG, Fuller OM (1985) Effect of colloidal materials on cellulase production by Trichoderma reesei Rut-C30. Appl Environ Microbiol 49(4):934–938
Emtiazi G, Pooyan M, Shamalnasab M (2007) Cellulase activities in nitrogen fixing Paenibacillus sp. isolated from soil in N-free media. World J Agri Sci 3(5):602–608
Fagan MJ, Saier MHJ (1994) P-type ATPases of eukaryotes and bacteria: sequence comparisons and construction of phylogenetic trees. J Mol Evol 38(1):57–99
Fath MJ, Kolter R (1993) ABC-transporters: bacterial exporters. Microbiol Rev 57(4):995–1017
Garrity GM, Johnson KL, Bell J, Searles D (2002) Bergey’s manual of systematic bacteriology: taxonomic outline of the prokaryotes, 2nd edn. Eds. Release 3.0 July 2002. Springer-Verlag, New York
Hakamada Y, Koike K, Yoshimatsu T, Mori H, Kobayashi T, Ito S (1997) Thermostable alkaline cellulase from an alkaliphilic isolate Bacillus sp. KSM-S237. Extremophiles 1(3):151–156
Hamer G (2003) Solid waste treatment and disposal: effect on public health and environmental safety. Biotechnol Adv 22:71–79
Hernández A, Mellado RP, Martínez JL (1998) Metal accumulation and vanadium-induced multidrug resistance by environmental isolates of Escherichia hermannii and Enterobacter cloacae. Appl Environ Microbiol 64(11):4317–4320
Husain RSA, Thatheyus AJ, Ramya D (2013) Bioremoval of nickel using Pseudomonas fluorescens. Am J Microbiol Res 1(3):48–52
Immanuel G, Dhanusha R, Prema P, Palavesam A (2006) Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment. Int J Environ Sci Technol 3(1):25–34
Ito S (1997) Alkaline cellulases from alkaliphilic Bacillus: enzymatic properties, genetics and application to detergents. Extremophiles 1(2):61–66
Ito S, Shikata S, Ozaki K, Kawai S, Okamoto K, Inoue S, Takei A, Ohta YI, Satoh T (1989) Alkaline cellulase for laundry detergents production by Bacillus sp. KSM 635 and enzymatic properties. Agri Biol Chem 53:1275–1281
Kaewchai S, Prasertsan P (2002) Biosorption of heavy metal by thermotolerant polymer-producing bacterial cells and the bioflocculant. Songklanakarin J Sci Technol 24(3):421–430
Karim A, Nawaz MA, Aman A, Qader SAU (2014) Hyper production of cellulose degrading endo (1,4) β-d-glucanase from Bacillus licheniformis KIBGE-IB2. J Rad Res Appl Sci. doi:10.1016/j.jrras.2014.06.004
Kawai S, Okoshi H, Ozaki K, Shikata S, Ara K, Ito S (1988) Neutrophilic Bacillus strain KSM.522 that products an alkaline carboxy methyl cellulase. Agri Biotechnol Chem 52:1425–1431
Kongruang S, Han MJ, Breton CIG, Penner MH (2004) Quantitative analysis of cellulase-reducing ends. Appl Biochem Biotechnol 113–116:213–231
Krishna C (1999) Production of bacterial cellulases by solid state bioprocessing of banana wastes. Biores Technol J 69:231–239
Krishna KB, Varma A (1990) Endoglucanase production by entrapped Bacillus thermoalkalophilus. World J Microbiol Biotechnol 6(3):267–272
Lankinen P, Kähkönen MA, Rajasärkkä J, Virta M, Hatakka A (2011) The effect of nickel concentration on growth of litter-decomposing fungi, extracellular enzyme activities and toxicity in soil. Boreal Environ Res 16(3):229–239
Leung WC, Wong MF, Chua H, Lo W, Yu PH, Leung CK (2000) Removal and recovery of heavy metals by bacteria isolated from activated sludge treating industrial effluents and municipal wastewater. Water Sci Technol 41(12):233–240
Lynd LR, Weimer PJ, Van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology microbiology. Microbiol Mol Biol Rev 66:506–577
Mawadza C, Zvauya R (1996) Some factors affecting endo-β-1,4-glucanase production by two Bacillus strains isolated from Zimbabwean hot springs. J Basic Microbiol 36:177–185
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mingardon F, Chanal A, López-Contreras AM, Dray C, Bayer EA, Fierobe HP (2007) Incorporation of fungal cellulases in bacterial minicellulosomes yields viable, synergistically acting cellulolytic complexes. Appl Environ Microbiol 73(12):3822–3832
Nies DH (1999) Microbial heavy metals resistance. Appl Microbiol Biotechnol 51(6):730–750
Noel RK (1984) Bergey’s manual of systematic bacteriology, vol 1. Williams and Wilkins Awaverly Co, USA, p 409–602
Padmavathy V (2008) Biosorption of nickel (II) ions by baker’s yeast: kinetic, thermodynamic and desorption studies. Bioresour Technol 99(8):3100–3109
Pettigrew CA, Johanson BN (1996) Testing the biodegradability of synthetic polymeric materials in solid waste. In: Palmisano AC, Barlaz MA (eds) Microbiology of solid waste. CRC Press Inc, Florida, pp 71–104
Ragsdale SW (1998) Nickel biochemistry. Curr Opin Chem Biol 2:208–215
Sadhu S, Saha P, Sen SK, Mayilraj S, Maiti TK (2013) Production, purification and characterization of a novel thermotolerant endoglucanase (CMCase) from strain isolated from cow dung. Springer plus 2:1–10
Schloss PD, Hay AG, Wilson DB, Gossett JM, Walker LP (2005) Quantifying bacterial population dynamics in compost using 16S rRNA gene probes. Appl Microbiol Biotechnol 66:457–463
Sethi S, Datta A, Gupta BL, Gupta S (2013) Optimization of cellulase production from bacteria isolated from soil. ISRN Biotechnol 2013:1–7
Silva R, Lago ES, Merheb CW, Macchione MM, Park YK, Gomes E (2005) Production of xylanase and CMCase on solid state fermentation in different residues by Thermoascus aurantiacus miehe. Brazilian J Microbiol 36:235–241
Smith RL, Maguire ME (1995) Distribution of the CorA Mg2+ transport system in gram-negative bacteria. J Bacteriol 177(6):1638–1640
Sneath P, Mair NS, Sharpe EM (1986) Bergey’s manual of systematic bacteriology, vol 2. Williams and Wilkins Awaverly Co, USA
Sternberg D, Mandels GR (1979) Induction of cellulolytic enzymes in T. reesei by sophorose. J Bacteriol 139:761–769
Stutzenberger FJ (1971) Cellulase production by Thermomonospora curvata isolated from municipal solid waste compost. Appl Microbiol 22:147–152
Sujarittanonta S, Sherrard JH (1981) Activated sludge nickel toxicity studies. J Water Poll Control Fed 53(8):1314–1322
Tao T, Snavely MD, Farr SG, Maguire ME (1995) Magnesium transport in Salmonella typhimurium: mgtA encodes a P-type ATPase and is regulated by Mg2+ in a manner similar of the mgt B P-type ATPase. J Bacteriol 177(10):2654–2662
Ten LN, Im WT, Kim MK, Kang MS, Lee ST (2004) Development of a plate technique for screening of polysaccharide-degrading microorganism by using a mixture of insoluble chromogenic substances. J Microbiol Meth 56:375–382
Wang YS, Byrd CS, Barlaz MA (1994) Anaerobic biodegradability of cellulose and hemicellulose in excavated refuse samples. J Indus Microbiol 13:147–153
Wattt RK, Ludden PW (1999) Nickel binding proteins. Cell Mol Life Sci 56:604–625
Zhang YH, Lynd LR (2004) Toward an aggregated understanding of enzymatic hydrolysis of cellulose. Non complexed cellulose systems. Biotechnol Bioeng 88:797–824
Zhang YH, Lynd LR (2005) Determination of the number average degree of polymerization cellodextrans and cellulose. Biomacro-molecules 6:1510–1515
Zhang YHP, Himmel ME, Mielenz JR (2006) Outlook for cellulase improvement: screening and selection strategies. Biotechnol Adv 24:452–481
Acknowledgments
This work was conducted at Taiz University (TU)-Yemen. I gratefully thank TU for their technical assistance and academic staff. Many thanks for Mr. Jamil Ali Saeed Al-Gheethi for his support during the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Al-Gheethi, A.A.S. Recycling of sewage sludge as production medium for cellulase by a Bacillus megaterium strain. Int J Recycl Org Waste Agricult 4, 105–119 (2015). https://doi.org/10.1007/s40093-015-0090-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-015-0090-6