Abstract
Purpose
Effective vermicomposting of Salvinia natans is a good alternative for protecting wetlands damaged by the weed due to the vast potential for use of the product vermicompost in agriculture, but the presence of toxic metals in the weeds may deter the usage.
Methods
Research was carried out on the physico-chemical and biological characteristics as well as bioavailability and leachability of nutrients and heavy metals (Zn, Cu, Mn, Fe, Ni, Pb, Cd and Cr) during vermicomposting of S. natans mixed with cattle manure and sawdust in five different combinations [trial 1 (eight S. natans: one cattle manure: one sawdust), trial 2 (seven S. natans: two cattle manure: one sawdust), trial 3 (six S. natans: three cattle manure: one sawdust), trial 4 (five S. natans: four cattle manure: one sawdust) and trial 5 (ten S. natans: 0 cattle manure: 0 sawdust)] for 45 days with Eisenia fetida earthworm.
Results
The highest growth of earthworms was in trial 4, having the highest (40 %) cattle manure. Trial 4 also indicated the highest reduction of volatile solids (38.6 %) and soluble BOD (82.3 %). The water-soluble forms of all nutrients were increased significantly. The highly bioavailable water-soluble and DTPA-extractable forms of heavy metals were reduced favourably after the process. The toxicity characteristic leaching procedure (TCLP) test indicated that leachable heavy metals of the vermicomposts were also reduced and were within the threshold limits for agricultural applications.
Conclusions
Eisenia fetida was very effective for reduction of bioavailable and leachable forms of selected heavy metals, and the TCLP test confirmed that the vermicompost was not hazardous for soil applications. The potential of the earthworms to increase the available nutrients, but mitigate the metal toxicity during vermicomposting of S. natans will be useful for sustainable land renovation practices.
Similar content being viewed by others
Introduction
The ecology of the wetlands is often damaged by proliferation of aquatic weeds in their water zones. Weeds interfere with the primary production of wetlands and impact severely on the biodiversity by out-competing native plants and degrading the habitat (DEC 2012). The floating water moss Salvinia natans is an invasive dominant colonizing aquatic fern species, which prevails over the aquatic ecosystem by its rapid growth and displacement of native plants that provide food and habitat for the native animals and waterfowl (Galka and Szmeja 2012). The growth and spread of Salvinia natans may be enormous to the extent of 45 % per day in nutrient-rich water bodies (Blackman 1960). The high viability of spores associated with rapid vegetative propagation is responsible for the spread of the weed producing vast thick mats and, with the impending global warming, the weed will create havoc to water bodies at the expense of submerged exotic plants in many parts of the world (Netten et al. 2010). The dense mats have a negative effect on the functioning and biodiversity of the freshwater ecosystems due to hampered photosynthesis, reduced gas exchange at the air–water interface and increased consumption of oxygen by microbial decomposition. The decayed dead weeds increase the nutrient level in the water, leading to the problem of eutrophication (Gupta et al. 2007). S. natans also covers vast free water zones of the Loktak Lake (24°25′–24°42′N; 93°46′–93°55′E) of Manipur, northeast India. The lake is the largest freshwater lake of the region and plays an important role in providing regional ecological and economic security by supporting valuable biodiversity or heterogeneity (Trisal and Manihar 2004).
Generation of quality composts through composting or vermicomposting of S. natans and subsequent land application of the composts can solve the problems of (1) uncontrolled growth of the weed in the water body and (2) soil infertility, thereby reducing the use of synthetic fertilizers (Singh and Kalamdhad 2013). The pile composting of S. natans has been investigated already by the authors (Singh et al. 2015). In case of vermicomposting, the earthworms ingest, grind and digest organic waste into vermicompost with relatively higher concentrations of plant nutrients, microbial population, soil enzymes and humic acids due to the joint action of earthworms and microorganisms (Khwairakpam and Bhargava 2009). Vermicomposting is a bio-oxidative process where the microorganisms, both in the gut of the earthworms and in the feedstock, are responsible for the biochemical degradation of the organic matter while the earthworms fragment the substrate, thereby increasing the surface area exposed to the microorganisms. Hence, the role of the earthworms is to directly or indirectly modify the physical and chemical properties of the substrates (Fornes et al. 2012).
Salvinia natans are highly effective in removing water pollutants and heavy metals and there is a possibility of the presence of heavy metals in their biomass. Therefore, the application of the resulting composts has the potential to cause adverse effects on the environment (Dhir and Srivastava 2011; Kumari and Tripathi 2014). High and excessive accumulation of heavy metals in the soil, their uptake by plants, successive accumulation in human tissues and bio-magnifications through the food chain are the main concerns for human health and environment (Wong and Selvam 2006; Iwegbue et al. 2007; Chiroma et al. 2012). The heavy metals exert toxic effects on soil microorganisms, resulting in the change of diversity, population and overall activity of the soil microbial communities (Ashraf and Ali 2007). The bio-available and leachable heavy metals, which are highly toxic due to their mobility, are of major concern to the environment (Liu et al. 2007). Bioavailable forms of heavy metals may be: (1) water soluble that are the most biologically active and have the highest potential for contaminating the food chain as well as surface and groundwater (Iwegbue et al. 2007); (2) diethylenetriaminepentaacetic acid (DTPA) extractable that are also plant available metals in soil at regular or higher concentrations (Samuel et al. 2013). DTPA is a chelating agent that mimics plant uptake of heavy metals by extracting carbonate-bound and organically bound metal fractions in calcareous soils (Bragato et al. 1998; Fang and Wong 1999; Fuentes et al. 2006; Walter et al. 2006); and (3) leachable that are the mobile forms of the metals associated with the movement of organic and inorganic analytes present in liquid, solid and multi-phasic wastes and determined through toxicity characteristics leaching procedure (TCLP) tests (USEPA 1992).
Earthworms are reported to reduce the possible toxic effects of heavy metals by consuming them during physiological metabolism (Jain et al. 2004; Suthar et al. 2014). Much work has been carried out on the bioavailability of heavy metals during vermicomposting of weeds such as water hyacinth (Singh and Kalamdhad 2013), but none has been reported for S. natans weed. A number of earthworm species such as Eisenia fetida, Eisenia andrei, Eudrilus eugeniae and Prionyx excavates have been studied for their potential to degrade the organic mass, and the epigeic earthworm E. fetida was found to be the best species for vermicomposting because of its wide range of tolerance and capability of producing good vermicompost (Edwards 1998; Sinha et al. 2002; Suthar 2007). The objective of the present study was to determine the physico-chemical transformations and assess the bioavailability and leachability of heavy metals of the vermicompost during vermicomposting of S. natans blended with cattle manure and sawdust in different combinations.
Materials and methods
Feedstock materials
Salvinia natans was collected from the Loktak Lake near Thanga Village, Bishnupur District, Manipur, India, and brought to the lakeside by local boatmen. The biomass was then transported to the vermicompost laboratory of the Indian Institute of Technology Guwahati (IITG), India. Cattle manure was obtained from the dairy farm near the IITG campus, North Guwahati, and sawdust was purchased from the nearby saw mill at Amingao, North Guwahati. The S. natans biomass was prepared for vermicomposting through cutting/shredding (maximum size restricted to 10 mm to provide better aeration and moisture control) and uniform premixing with cattle manure and sawdust.
Earthworms
The epigeic earthworm, Eisenia fetida, was selected for the experiment as it was reported to be effectively used worldwide for vermicomposting of organic wastes (Edwards 1998). The earthworms were brought from the Central Plantation Crops Research Institute (CPCRI), Indian Council of Agricultural Research, Kahikuchi, Guwahati, Assam. Perspex bin size of 450 × 300 × 450 mm was fabricated for culturing the earthworms in the laboratory. 16 holes (10 mm diameter) were drilled at equal spacing along the longer sides and at the bottom of the bin for aeration and drainage purposes. Before the addition of the culturing media and the earthworms, 10 cm-thick bedding for the earthworms was prepared from partially degraded chopped hay (about 50 mm), cattle manure, banana pulp (chopped about 50 mm) and tree leaves. The bedding was then watered to keep it moist to facilitate breathing of the earthworms. The earthworm species were then added with partially degraded cow dung as culturing media (Singh and Kalamdhad 2013).
Experimental set up
The experiments were conducted in triplicate at room temperature in locally made curved shaped bamboo containers (reactor) of dimensions 150 mm top radius and 100 mm depth to facilitate better ventilation for the earthworms (Fig. 1). The reactors were designed for 2.5 kg of the substrates and approximately 100 g live weight of earthworms (200 nos. of clitellated and young non-clitellated E. fetida) was randomly picked from the perspex bin culture and introduced gently into the substrates from the top. The earthworm biomass weight was decided considering that earthworms can consume materials approximately half their body weight per day under favourable conditions (Haimi and Huhta 1986). Five trials were run in triplicate with the different mixtures of S. natans, cattle manure and sawdust as detailed below:
Diagram of a bamboo vermireactor (Sonowal et al. 2014)
Trial 1 (T1): S. natans (2.0 kg) + cattle manure (0.25 kg) + sawdust (0.25 kg) + E. fetida (approx. 0.1 kg)
Trial 2 (T2): S. natans (1.75 kg) + cattle manure (0.50 kg) + sawdust (0.25 kg) + E. fetida (approx. 0.1 kg)
Trial 3 (T3): S. natans (1.50 kg) + cattle manure (0.75 kg) + sawdust (0.25 kg) + E. fetida (approx. 0.1 kg)
Trial 4 (T4): S. natans (1.25 kg) + cattle manure (1.00 kg) + sawdust (0.25 kg) + E. fetida (approx. 0.1 kg)
Trial 5 (T5): S. natans (2.5 kg) + cattle manure (0 kg) + sawdust (0 kg) + E. fetida (approx. 0.1 kg)
The optimum moisture content is one of the most important requirements for the earthworms throughout the vermicomposting process. Excess moisture content may create anaerobic conditions which may be fatal to the earthworms (Garg and Gupta 2011). All the containers were kept in the dark under identical ambient conditions (room temperature 25 ± 3 °C). The moisture level was maintained at about 50–60 % throughout the study period by periodic sprinkling of adequate quantity of tap (potable) water whenever required. To prevent moisture loss, the reactors were covered with gunny bags. The mixtures were manually turned at every 15th day to provide suitable aeration to the earthworms.
Sampling and analysis of earthworm biomass and physico-chemical parameters
The earthworms and hatchings were separated by light separation and hand sorting method, and the earthworm biomass was weighed. The cocoons were sorted manually. Wet substrate (free of earthworms, hatchlings and cocoons) were collected from the reactors on day 0 (before inoculation of earthworms) and on the 15th, 30th and 45th day of the vermicomposting period after earthworm separation and stored immediately at 4 °C for biological analysis. Sub-samples were oven dried, ground to pass through a 0.2 mm sieve and stored in plastic bags for further analysis of the physico-chemical parameters.
Each sample was analysed for pH, electrical conductivity (EC) (from 1:10 w/v waste: water extract) and organic matter in terms of volatile solids (VS) (loss on ignition at 550 °C for 2 h in muffle furnace). Total Kjeldahl nitrogen (TKN) was analysed using the Kjeldahl method, ammoniacal nitrogen (NH4-N) using KCl extraction, total phosphorous (TP) and available phosphorus (AP) (by acid digest) using the stannous chloride method. The flame photometer (Systronic 128) was used for analysis of sodium (Na), potassium (K) and calcium (Ca) concentration, whereas the atomic absorption spectrometer (Varian Spectra 55B) was used for analysis of magnesium (Mg), zinc (Zn), copper (Cu), manganese (Mn), iron (Fe), nickel (Ni), lead (Pb), cadmium (Cd) and chromium (Cr) concentrations after digestion of 0.2 g dry sample with 10 mL mixture of 5H2SO4 and 1HClO4 in a block digestion system (Pelican Equipments Chennai-India) for 2 h at 300 °C (APHA 2005). Water-soluble portions were determined after the extraction of 2.5 g sample with 50 mL of distilled water at room temperature in a shaker at 100 rpm for 2 h (Singh and Kalamdhad 2013).
Soluble bio-chemical oxygen demand (BOD) and soluble chemical oxygen demand (COD) were determined from the supernatant of the blended mixture of 10 g wet sample in 100 mL deionized water by the dilution and dichromate method, respectively (APHA 2005). The water-soluble (WS) portions were determined after extraction of 2.5 g sample with 50 mL of distilled water at room temperature in a shaker at 100 rpm for 2 h (Singh and Kalamdhad 2013). Diethylenetriaminepentaacetic acid (DTPA) extraction of the metals was carried out by mechanically shaking 4 g ground sample (screened through 0.22 mm sieve) with 40 mL of 0.005 M DTPA, 0.01 M CaCl2 and 0.1 M (triethanolamine) buffered to pH 7.3 at 100 rpm (Guan et al. 2011). The standard Toxicity characteristic leaching procedure (TCLP) test was performed to determine the leachable portions (USEPA 1992). 5 g compost sample (size less than 9.5 mm) with 100 mL of acetic acid at pH 4.93 ± 0.05 (pH adjusted with 1 N NaOH) was taken in a 125 mL reagent bottle and kept at room temperature for 18 h in a shaker at 30 ± 2 rpm. The suspensions were centrifuged for 5 min at 10,000 rpm, filtered through Whatman filter paper no. 42 and the filtrate stored in a plastic reagent bottle at 4 °C for analysis of the selected heavy metals.
The results reported are the means of three replicates. Analysis of variance (ANOVA) test using the SPSS software was carried out for each parameter on all five trials to determine the significance of variations.
Results and discussion
The initial characteristics of the substrates are shown in Table 1. Cattle manure is rich in soluble BOD (4560 mg/kg) and COD (11,600 mg/kg), indicating good source of easily metabolizable organic matter. The S. natans sample contains 76.8 % VS and is also rich in nutrients. The initial pH of the S. natans, cattle manure and sawdust ranges from 5.7 to 7.0 and is within the range (5.5–8.5), suitable for activity of the earthworms and microorganisms (Yadav and Garg 2011). The S. natans indicated the presence of the following heavy metals in order of their concentrations: Fe > Mn > Pb > Ni > Zn > Cr > Cu > Cd.
E. fetida biomass
Figure 2a illustrates the changes in the number of earthworms, hatchings and cocoons. The total number of earthworms (clitellated and young non-clitellated) initially decreased in all trials. The number of earthworms was found to increase on day 45 in T2 (206 ± 1), T3 (235 ± 2) and T4 (242 ± 4) (Fig. 2a). No mortality was indicated in T3 and T4 during the process. The T4 substrate had the highest proportion of cattle manure, containing easily metabolizable organic matter and non-assimilated carbohydrates essential for the growth and reproduction of earthworms (Gupta et al. 2007). The growth of the earthworms is also related to the palatability, nutrient pool and microbial populations of the feed (Flegel and Schrader 2000; Suthar 2009). The high mortality in T5 indicates that S. natans without proper amendments is not suitable for vermicomposting. The cocoon population was first seen in trials during the monitoring of the reactors on day 15 of the process. The highest number of cocoons was also observed on day 30 in T4 (105 ± 5), followed by T3 (90 ± 1). The decrease in the total number of cocoons on day 45 in T2, T3 and T4 was due to the hatching of the cocoons. The production of cocoons in different feed mixtures is also related to the biochemical quality of the feed and the microbial mass and decomposition activities (Flack and Hartenstein 1984; Suthar 2007). Few new hatchings were first observed during monitoring on day 30 in all trials, and the highest number of hatchings was in T4 (203 ± 5) followed by T3 (173 ± 6) on day 45. The highest increase of total biomass was in T4 (302.2 g), followed by T3 (232.0 g) on day 45 (Fig. 2b). In T4, the biomass growth rates from 0 to 15, 15 to 30 and 30 to 45 days were 4.4, 1.7 and 7.4 g/day. In T3, the biomass growth rates from 0 to 15, 15 to 30 and 30 to 45 days were 2.8, 1.0 and 5.0 g/day. The decrease in the biomass growth rate from 15 to 30 days was possibly due to the energy consumed by the adult earthworms for production of cocoons, which otherwise would have been utilized for tissue growth (Chaudhari and Bhattacharjee 2002). The negative peak of the biomass growth rate of the control trial T5 without cattle manure on day 15 was due to the mortality that occurred during the acclimatization period of the earthworms for the substrates. The rise in the biomass growth rate on day 45 was due to the hatchings and their rapid growth. The average total biomass growth rate after the vermicomposting process was highest in T4 (4.5 g/day) followed by T3 (2.9 g/day). Therefore, the growth and fecundity were dependent on the cattle manure proportions of the substrates. The rate of growth of the earthworms and their increase in population as well as fecundity are indicators of the suitability for vermicomposting of the substrates (Chaudhari and Bhattacharjee 2002). ANOVA test indicates that the variations of the number of earthworms (F = 7.2, P = 0.0 < 0.01), cocoons (F = 10.5, P = 0.00 < 0.01) and hatchings (F = 3.0, P = 0.04 < 0.05) and the total biomass (F = 8.8, P = 0.0 < 0.01) are significant for the five trials.
Physico-chemical transformations
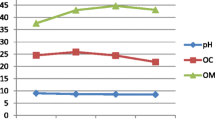
The pH in all trials increased after the vermicomposting process (Fig. 3a). The pH of T1, T2, T3, T4 and T5 increased from 6.13, 6.40, 6.50, 6.56 and 6.00 to 7.11, 7.25, 7.43, 7.61 and 7.02 on day 45, respectively. The pH of T1, T2 and T4 initially decreased to 6.10, 6.30 and 6.48 on day 15, respectively. The initial decrease of pH in some of the trials could be attributed to the production of organic acid by the associated microbial decomposition during the vermicomposting process, which lowers the pH (Suthar 2007). Different authors have different views about the variation of pH values during composting. The decomposition of organic matter leads to the formation of ammonium (NH4 +) ions which increases the pH value (Pramanik et al. 2007). The inconsistency in the findings is because different substrates result in the production of different intermediate species and different wastes showing different pH behaviours (Gupta and Garg 2008). The gradual increase in pH during vermicomposting was corroborated by Datar et al. (1997), Hait and Tare (2010), Kaur et al. (2010) and Singh and Kalamdhad (2013). Earthworms selectively increased the populations of catabolically more active microbes (Aira et al. 2007) and, in the process, the degradation of short chain fatty acids and precipitation of calcium carbonate might have increased the pH during the process (Tognetti et al. 2005). It must be borne in mind that the amorphous calcium carbonate in bio-minerals fulfils the functions of skeletal growth and maintenance in higher organisms which is not relevant in the case of earthworms. The secretion of calcium carbonate appears to be related to pH regulation, with calcium carbonate precipitating when HCO3 − ions are in excess of those required to buffer tissue fluid pH (Versteegh et al., 2014). Also, the excess organic nitrogen that was not entailed by microbes and released as ammonia might have dissolved in the moisture of the substrates and increased the pH of the vermicompost (Vig et al. 2011). The variation of pH amongst the trials was highly significant (F = 34.5, P = 0.0 < 0.01).
The EC of T1 and T2 increased from 3.20 and 3.35 to 3.44 and 3.52 dS/m on day 45, respectively (Fig. 3b). The increase in EC was due to loss of organic matter and release of different mineral salts in available forms such as phosphate, ammonium and potassium. (Lim et al. 2014). Some of these ions are essential for plant growth (K+, Ca2+, Mg2+, etc.), while others are often undesirable (Na+, NH4 +, heavy metal ions, etc.). The EC of T5 decreased from 3.45 to 3.30 dS/m during the process, indicating the slow mineralization process of the T5 feedstock. The EC of T3 and T4 after an initial increase from 3.30 and 3.25 dS/m to 3.58 and 3.76 dS/m decreased to 2.45 and 2.15 dS/m, respectively. The precipitation of mineral salts could be the reason for the final decrease in EC and it corroborated with the increased pH. The change in the availability of cations or anions with pH may also affect metal mobility and availability through competitive sorption and complexation reactions (Sizmur and Hodson 2009). EC reflects the salinity of the composting product and its suitability for plant growth. High EC in the final product is undesirable, because it will inhibit plant rooting and also reduce the transportation of water and nutrients to the plants (Fang and Wong 1999; Chiang et al. 2007). ANOVA test shows no significant difference in the EC amongst all trials (F = 1.4, P = 0.27 > 0.05). However, the decrease in EC on day 45 for all trials was significant (F = 3.2, P = 0.04 < 0.05).
The VS concentration is an indicator of organic matter content and decreased during the vermicomposting process (Fig. 3c). The metabolic activity of the earthworms resulting in assimilation of carbohydrates/other polysaccharides from the substrates and the microbial respiration of the active microorganisms thriving in the microclimatic conditions promoted by the earthworms degraded the organic components, resulting in the loss of carbon in the form of CO2 and subsequent mineralization (Vig et al. 2011). The loss of VS in the trials was in the order T4 (38.6 %) > T3 (35.6 %) > T2 (26.5 %) > T1 (16.0 %) > T5 (5.8 %). T4 had the highest proportion of cattle manure containing fungal stains and greater population of other microbes such as bacteria, protozoa, nematodes, fungi and actinomycetes that played an important role in organic matter decomposition by providing extracellular enzymes in the reactors (Suthar et al., 2014). The results were consistent with the findings during vermicomposting of water hyacinth (Singh and Kalamdhad 2013). The reduction trend in VS was also in agreement with the growth profile of E. fetida (Fig. 2). ANOVA analysis shows significant difference in the reduction of VS amongst all trials (F = 16.3, P = 0.00 < 0.05).
Soluble BOD analysis is a measure of the readily degradable organic matter of the compost mixture and is an important parameter in the analysis of the stability of the compost, as the readily bioavailable compounds are subjected to rapid aerobic microbial degradation. Oxygen consumption will continue if soluble BOD is high, indicating unstable compost. During land applications of such composts for crops, the biological processes can continue and strip nutrients from soil (Wang et al. 2004). All trials showed reduced soluble BOD after the process. The order of soluble BOD reduction was T4 (82.3 %) > T3 (79.1 %) > T2 (76.1 %) > T1 (72.7 %) > T5 (66.7 %).
Nutrients
The TKN increased in all trials during the vermicomposting process from 1.95, 1.88, 1.81, 1.74 and 2.20 % to 2.41, 2.43, 2.49, 2.63 and 2.66 % in T1, T2, T3, T4 and T5, respectively (Table 2). The highest increase was in T4 (51.0 %), followed by trial 3 (37.3 %). The concentration of TP gradually increased due to the mineralization and mobilization of phosphorous by bacterial and faecal phosphatase activity of the earthworms (Khwairakpam and Bhargava 2009). TP of T1, T2, T3, T4 and T5 increased from 2.02, 2.13, 2.24, 2.35 and 2.10 g/kg to 3.20, 3.55, 4.00, 4.38 and 2.91 g/kg, and the highest increase was in T4 (86.2 %) followed by T3 (78.3 %).
The nutrients (Na, K, Ca and Mg) are needed in minimal amounts for earthworm metabolism. K, Ca and Mg are also essential for plant growth, but Na in high concentration is often undesirable. Na is, however, considered as a functional plant nutrient by some authors (Subbarao et al. 2003). Ca and Na in the form of oxides or hydroxides or carbonates in composts when applied to soil may counteract soil acidification, making soil nutrients more available to plants. The total minerals in all trials increased after the process on day 45 (Table 2). The highest increase of Na (84.6 %), K (48.9 %), Ca (65.4 %) and Mg (34.2 %) was in T4, followed by T3. The result was in agreement with the highest growth of earthworms and highest reduction of VS in T4. The microflora present in the gut of earthworms, the associated microbial activity during the vermicomposting enhancing mineralization and the net loss in dry mass increased the concentration of the nutrients of the vermicompost (Suthar 2007; Hait and Tare 2010). The variations of total nutrients during the composting process were significant amongst all trials (TKN: F = 13.1, P = 0.0 < 0.01; TP: F = 14.7, P = 0.0 < 0.01; Na: F = 5.3, P = 0.004 < 0.05; K: F = 238, P = 0.00 < 0.01; Ca: F = 3.2, P = 0.04 < 0.05; Mg: F = 3.5, P = 0.04 < 0.05).
The water-soluble (WS) nutrients are the most available forms for the plants and they increased during the process also due to net loss of dry mass. The feeding action of the earthworms and the presence of a large number of micro-organisms and enzymes during vermicomposting transformed the insoluble plant nutrients into more soluble and available forms, thereby increasing their concentrations in the vermicomposts (Aira et al. 2007; Liu et al. 2012). When the organic substrates pass through the gut of the worm, some fraction of organic minerals are converted into more available species of nutrients (i.e. exchangeable forms) due to the action of endogenic and/or exogenic enzymes (Suthar 2010). The highest increase of WS Na (77.5 %), WS K (66.9 %), WS Ca (64.0 %) and WS Mg (72.7 %) was also in T4. In evaluating the contents of nutrients as well as undesirable plant salts, it is to be borne in mind that compost is primarily an organic soil conditioner and the use of pure compost is not recommended (CCME 2005). The variations of water-soluble nutrients during the composting process were significant amongst all trials for Na: F = 6.4, P = 0.00 < 0.01; K: F = 61, P = 0.00 < 0.01; Mg: F = 7.4, P = 0.00 < 0.01). The water-soluble nutrients of all trials increased significantly on day 45.
Total heavy metals concentration
The variation in total heavy metals (Zn, Cu, Mn, Fe, Ni, Pb, Cd and Cr) and their water-soluble (WS) and DTPA-extractable portions during the vermicomposting are shown at Table 3. The heavy metals are important ingredients for plants, animals and humans, but their higher content is known to have toxic effects. The total concentration of all selected heavy metals in the final vermicompost increased [Zn (14.9–19.7 %), Cu (8.7–19.7 %), Mn (14.8–23.2 %), Fe (11.8–21.9 %), Cr (15.9–31.3 %), Ni (10.5–19.2 %), Pb (2.1–22.9 %) and Cd (3.4–13.7 %)] due to reduction in the weight and volume of the S. natans biomass (Vig et al. 2011). Increase in heavy metals’ concentration in the vermicompost of different wastes was also reported by Kaushik and Garg (2003). Even though net increase in all heavy metals was indicated in all trials, Table 4 shows that there were downward trends in T2, T3 and T4 for Cr, Ni, Cd and Pb on day 45. There were also downward trends for Cu and Fe in T3 and T4 on day 45. The reason for the downward trend might be the bio-accumulation of heavy metals in the earthworm tissues (Suthar et al. 2014) The variation in Zn (F = 82, P = 0.0 < 0.01), Cu (F = 39, P = 0.0 < 0.01), Mn (F = 76, P = 0.0 < 0.01), Fe (F = 194, P = 0.0 < 0.01), Ni (F = 7.5, P = 0.0 < 0.01), Pb (F = 6.1, P = 0.0 < 0.01), Cd (F = 9.3, P = 0.0 < 0.01) and Cr (F = 3.8, P = 0.02 < 0.05) concentrations in different trials were significant. The order of total metal concentration of the vermicompost was Fe > Mn > Pb > Ni > Zn > Cr > Cd > Cu.
Water-soluble heavy metals
Evaluation of water-soluble heavy metals is necessary in the final vermicompost before agronomic application, because these metal fractions are biologically dynamic and have the highest potential of contaminating the food chain (Iwegbue et al. 2007; Hait and Tare 2012). Water solubility of metals was reduced in the range of about 13.5–27.5 % for Zn, 6.6–35.1 % for Cu, 9.7–36.3 % for Mn, 3.3–37.8 % for Fe, 23.6–80.0 % for Pb and 23.2–50.6 % for Cr during the vermicomposting process (Table 4). The order of water-soluble heavy metals after vermicomposting was: Fe > Mn > Cr > Zn > Cu > Pb. Water-soluble concentrations of Ni and Cd were not detected during the vermicomposting of S. natans. ANOVA test indicated that the variation of water-soluble heavy metals was found to be statistically different in all the trials during the vermicomposting process (Zn: F = 172, P = 0.0 < 0.01; Cu: F = 119, P = 0.0 < 0.01; Mn: F = 77, P = 0.0 < 0.01; Fe: F = 44, P = 0.0 < 0.01; Cr: F = 544, P = 0.0 < 0.01; Pb: F = 54, P = 0.0 < 0.01).
Considerable reductions in concentrations of water-soluble Cu, Zn and Cr during vermicomposting of sewage sludge were reported by Hait and Tare (2012). The pH and microbial activity of the gut of the earthworm were enhanced during the passage of the partially digested organic matter. Consequently, the possibilities of binding of metals to the more soluble ions and carbonates increased in the ingested material. According to Goswami et al. (2013), earthworms can bio-accumulate heavy metal ions by forming organo-metallic complexes in their intestines, which reduces the solubility of the heavy metals. Therefore, the bioaccumulation of water-soluble fraction of metals by E. fetida and the formation of organometallic complexes decreased the concentration of water-soluble form of heavy metals (Suthar 2009; Singh and Kalamdhad 2013). Earthworms decrease the mobility and bioavailability of heavy metals during vermicomposting through two major types of cellular adaptations: binding of metals to nuclear proteins forming inclusion nuclear bodies; and the cytoplasmic process involving synthesis of a specific metal binding protein, metallothionein, within the chloragogenous tissue (Cherian and Nordberg 1983; Hait and Tare 2012).
Plant-available heavy metals
The toxicity of heavy metals in the final product is dependent on the metal mobility in free form, the route of uptake mechanism and the bioavailability if it is accumulated in plants (Vig et al. 2011). DTPA is a chelating agent that mimics plant uptake of heavy metals and generally, metal fraction extracted with DTPA can be considered as potentially available for plants (Chiang et al. 2007). The DTPA extractability of metals was reduced in the range of about 8.3–31.0 % for Zn, 11.8–39.2 % for Cu, 7.0–18.2 % for Mn, 18.4–37.2 % for Fe, 35.9–82.7 % for Pb, 43.0–78.9 % for Ni and 17.4–61.9 % for Cr during the vermicomposting process. The order of DTPA-extractable heavy metal concentrations of the vermicompost was: Fe > Mn > Zn > Cr > Pb > Ni > Cu. The DTPA-extractable Cd was not detected during the vermicomposting of S. natans. The conversion of highly toxic Cr(VI) to nontoxic form Cr(III) through the metabolic process in the mitochondrial and cytoplasmic fractions was reported during vermicomposting with E. fetida (Jain et al. 2004). The reduction of DTPA-extractable metals during vermicomposting has also been reported by other researchers (Suthar 2009; Singh and Kalamdhad 2013). The interaction of the humic acid with metal is one of the main factors affecting the partitioning of heavy metals during the process (Hait and Tare 2012). The DTPA-extractable heavy metals were found to be statistically different (Zn: F = 47, P = 0.0 < 0.01; Cu: F = 47, P = 0.0 < 0.01; Mn: F = 6.5, P = 0.0 < 0.01; Fe: F = 45, P = 0.0 < 0.01; Cr: F = 106, P = 0.0 < 0.01; Pb: F = 272, P = 0.0 < 0.01) amongst all the trials during the vermicomposting process.
Leachable heavy metals
The TCLP test is designed to determine the mobility of both organic and inorganic analytes present in liquid, solid and multiphasic wastes. The leachable fractions of the heavy metals are shown at Table 4. The leachability of heavy metals was reduced in the range of about 9.8–33.5 % for Zn, 15.1–35.3 % for Cu, 10.8–24.3 % for Mn, 12.2–32.6 % for Fe, 15.7–52.7 % for Ni, 23.0–69.0 % for Pb, 39.2–98.0 for Cd and 23.5–63.2 % for Cr during the vermicomposting process. The order of leachable heavy metals’ content of the vermicompost was: Fe > Mn > Ni > Zn > Cr > Pb > Cu > Cd. The variations in the leachability of heavy metals amongst all trials were significant during the process (Zn: F = 241, P = 0.0 < 0.01; Cu: F = 174, P = 0.0 < 0.01; Mn: F = 9.6, P = 0.0 < 0.01; Fe: F = 41, P = 0.0 < 0.01; Cr: F = 161, P = 0.0 < 0.01; Pb: F = 58, P = 0.0 < 0.01).
The accumulation of metals in the earthworm tissues might be the reason for reduction of leachable concentration of metals during vermicomposting of S. natans. Similar results were also reported by Jain et al. (2004) and Singh and Kalamdhad (2013). The evidence of cutaneous absorption of metals by earthworms was reported by Suthar (2009). The leachability of metals might have also reduced due to increase in pH and complexity of metal humic substances. An increase in the pH of vermicompost resulted in an increase of surface negative charge, which increased cationic adsorption, formation of metal hydroxyl species that have a greater affinity for adsorption sites than the metal cations, and precipitation of metal as metal hydroxides (Maity et al. 2008). Another reason for the reduction of leachable metal concentration during vermicomposting was the affinity of the metals with the functional groups –OH or –COO– of humic substances of the vermicompost (Kang et al. 2011). The threshold limits for heavy metal contamination are 20 mg/kg for Cd, 100 mg/kg for Cr and 100 mg/kg for Pb (US EPA method 1311, 1992). Therefore, the results of the TCLP test confirmed that the heavy metals’ concentrations in all trials were under the threshold limits for compost use for agriculture purposes.
Conclusion
The addition of appropriate cattle manure in the feed mixture enhanced the growth and production of the earthworms. Reduction of organic matter was related to the amount of cattle manure addition, as indicated by the highest reduction of VS in trial T4 (38.6 %), followed by trial T3 (35.6 %). The performance of trial T4 was the best in terms of the highest reduction of VS, highest increase of the nutrients and the highest reduction of most of the bioavailable forms of heavy metals. With the appropriate proportion of cattle manure, E. fetida was effective in the reduction of water-soluble, DTPA-extractable and leachable heavy metals (Zn, Cu, Mn, Fe, Pb and Cr). The leachable concentration of heavy metals in all the trials was under the threshold limits. Ni and Cd were not detected in the water extract. The change in the order of concentrations of the bioavailable and leachable forms of heavy metals compared with the order of the total metal concentrations clearly indicated that the toxicology was dependent only on the bioavailable and leachable forms rather than the total metal concentrations.
References
Aira M, Monroy F, Dominguez J (2007) Earthworms strongly modify microbial biomass and activity triggering enzymatic activities during vermicomposting independently of the application rates of pig slurry. Sci Tot Environ 385(1–3):252–261. doi:10.1016/j.scitotenv.2007.06.031
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington DC
Ashraf R, Ali TA (2007) Effect of heavy metals on soil microbial community and mung seed germination. Pak J Bot 39(2):629–636
Blackman GE (1960) Responses to environmental factor by plants in the vegetative phase. In: Xarrow XM (ed) Growth of living system. Basic Books, New York, pp 525–556
Bragato G, Leita L, Figliolia A, Nobili M (1998) Effects of sewage sludge pre-treatment on microbial biomass and bioavailability of heavy metals. Soil Till Res 46(1–2):129–134. doi:10.1016/S0167-1987(98)80114-1
CCME (2005) Guidelines for compost quality. Canadian Council of Ministers for Environment, Ottawa
Chaudhari PS, Bhattacharjee G (2002) Capacity of various experimental diets to support biomass and reproduction of Perionyx excavates. Bioreour Technol 82(2):147–150. doi:10.1016/S0960-8524(01)00169-9
Cherian MG, Nordberg M (1983) Cellular adaptation in metal toxicology and metallothionein. Toxicology 28(1–2):1–15. doi:10.1016/0300-483X(83)90101-4
Chiang KY, Huang HJ, Chang CN (2007) Enhancement of heavy metal stabilization by different amendments during sewage sludge composting process. J Environ Eng Manage 17(4):249–256
Chiroma TM, Ebewele RO, Hymore FK (2012) Levels of heavy metals (Cu, Zn, Pb, Fe and Cr) in bushgreen and roselle irrigated with treated and untreated urban sewage water. Int Res J Environ Sci 1(4):50–55
Datar MT, Rao MN, Reddy S (1997) Vermicomposting- a technological option for solid waste management. J Solid Waste Technol Manage 24:89–93
DEC (2012) Wetland weeds. In: Bell T (ed) A guide to managing and restoring wetlands in Western Australia. Department of Environment and Conservation, Perth
Dhir B, Srivastava S (2011) Heavy metal removal from a multi-metal solution and wastewater by Salvinia natans. Ecol Eng 37(6):893–896. doi:10.1016/j.ecoleng.2011.01.007
Edwards CA (1998) The use of earthworms in the breakdown and management of organic wastes. In: Edwards CA (ed) Earthworm Ecology. CRC Press, Boca Raton, pp 327–354
Fang M, Wong JWC (1999) Effects of lime amendment on availability of heavy metals and maturation in sewage sludge composting. Environ Pollut 106(1):83–89. doi:10.1016/S0269-7491(99)00056-1
Flack FM, Hartenstein R (1984) Growth of the earthworm Eisenia foetida on microorganisms and cellulose. Soil Biol Biochem 16(5):491–495. doi:10.1016/0038-0717(84)90057-9
Flegel M, Schrader S (2000) Importance of food quality on selected enzyme activities in earthworm casts (Dendrobaena octaedra, Lumbricidae). Soil Biol Biochem 32(8–9):1191–1196. doi:10.1016/S0038-0717(00)00035-3
Fornes F, Mendoza-Hernández D, García-de-la-Fuente R, Abad M, Belda RM (2012) Composting versus vermicomposting: a comparative study of organic matter evolution through straight and combined processes. Bioresour Technol 118:296–305. doi:10.1016/j.biortech.2012.05.028
Fuentes A, Llorens M, Saez J, Aguilar MI, Marın ABP, Ortuno JF, Meseguer VF (2006) Ecotoxicity, phytotoxicity and extractability of heavy metals from different stabilised sewage sludges. Environ Pollut 143(2):355–360. doi:10.1016/j.envpol.2005.11.035
Galka A, Szmeja J (2012) Distribution, abundance and environmental conditions of the clonal aquatic fern Salvinia natans (L.) All. in the Vistula Delta (Baltic Sea Region). Bi Div Res Conserv 28:45–53. doi:10.2478/v10119-012-0020-7
Garg VK, Gupta R (2011) Optimization of cow dung spiked pre-consumer processing vegetable waste for vermicomposting using Eisenia fetida. Ecotoxicol Environ Saf 74(1):19–24. doi:10.1016/j.ecoenv.2010.09.015
Goswami L, Patel AK, Dutta G, Bhattacharyya P, Gogoi N, Bhattacharya SS (2013) Hazard remediation and recycling of tea industry and paper mill bottom ash through vermiconversion. Chemosphere 92(6):708–713. doi:10.1016/j.chemosphere.2013.04.066
Guan TX, He HB, Zhang XD, Bai Z (2011) Cu fractions, mobility and bioavailability in soil-wheat system after Cu-enriched livestock manure applications. Chemosphere 82(2):215–222. doi:10.1016/j.chemosphere.2010.10.018
Gupta R, Garg VK (2008) Stabilization of primary sewage sludge during vermicomposting. J Hazard Mater 153(3):1023–1030. doi:10.1016/j.jhazmat.2007.09.055
Gupta R, Mutiyar PK, Rawat NK, Saini MS, Garg VK (2007) Development of a water hyacinth based vermireactor using an epigeic earthworm Eisenia fetida. Bioresour Technol 98(13):2605–2610. doi:10.1016/j.biortech.2006.09.007
Haimi J, Huhta V (1986) Capacity of various organic residues to support adequate earthworm biomass for vermicomposting. Biol Fert Soils 2:23–27. doi:10.1007/BF00638957
Hait S, Tare V (2010) Vermistabilization of primary sewage sludge. Bioresour Technol 102(3):2812–2820. doi:10.1016/j.biortech.2010.10.031
Hait S, Tare V (2012) Transformation and availability of nutrients and heavy metals during integrated composting-vermicomposting of sewage sludges. Ecotoxicol Environ Saf 79(1):214–224. doi:10.1016/j.ecoenv.2012.01.004
Iwegbue CMA, Emuh FN, Isirimah NO, Egun AC (2007) Fractionation, characterization and speciation of heavy metals in composts and compost-amended soils. Afr J Biotechnol 6(2):067–078. doi:10.1002/chin.200748228
Jain K, Singh J, Chauhan LKS, Murthy RC, Gupta SK (2004) Modulation of flyash-induced genotoxicity in Vicia faba by vermicomposting. Ecotoxicol Environ Saf 59(1):89–94. doi:10.1016/j.ecoenv.2004.01.009
Kang J, Zhang Z, Wang JJ (2011) Influence of humic substances on bioavailability of Cu and Zn during sewage sludge composting. Bioresour Technol 102(17):8022–8026. doi:10.1016/j.biortech.2011.06.060
Kaur A, Singh J, Vig AP, Dhaliwal SS, Rup PJ (2010) Cocomposting with and without Eisenia fetida for conversion of toxic paper mill sludge to a soil conditioner. Bioresour Technol 101(21):8192–8198. doi:10.1016/j.biortech.2010.05.041
Kaushik P, Garg VK (2003) Vermicomposting of mixed solid textile mill sludge and cow dung with epigeic earthworm Eisenia fetida. Bioresour Technol 90(3):311–316. doi:10.1016/S0960-8524(03)00146-9
Khwairakpam M, Bhargava R (2009) Vermitechnology for sewage sludge recycling. J Hazard Mater 161:948–954. doi:10.1016/j.jhazmat.2008.04.088
Kumari M, Tripathi BD (2014) Effect of aeration and mixed culture of Eichhornia crassipes and Salvinia natans on removal of wastewater pollutants. Ecol Eng 62:48–53. doi:10.1016/j.ecoleng.2013.10.007
Lim PN, Wu TY, Clarke C (2014) Treatment and biotransformation of highly polluted agro-industrial wastewater from palm oil mill into vermicomposting using earthworms. J Agric Food Chem 62(3):691–698. doi:10.1021/jf404265f
Liu Y, Ma L, Li Y, Zheng L (2007) Evolution of heavy metal speciation during the aerobic composting process of sewage sludge. Chemosphere 67:1025–1032. doi:10.1016/j.chemosphere.2006.10.056
Liu J, Lu Z, Yang J, Xing M, Yu F, Guo M (2012) Effect of earthworms on the performance and microbial communities of excess sludge treatment process in vermifilter. Bioresour Technol 117:214–221. doi:10.1016/j.biortech.2012.04.096
Maity S, Padhy PK, Chaudhury S (2008) The role of earthworm Lampito mauritii (Kinberg) in amending lead and zinc treated soil. Bioresour Technol 99(15):7291–7298. doi:10.1016/j.biortech.2007.12.079
Netten JJC, Gertie HP, Arts GHP, Gylstra R, van Nes EH, Scheffer M, Roijackers RMM (2010) Effect of temperature and nutrients on the competition between free-floating Salvinia natans and submerged Elodea nuttallii in mesocosms. Fundamental Appl Limnology 177(2):125–132. doi:10.1127/1863-9135/2010/0177-0125
Pramanik P, Ghosh GK, Ghosal PK, Banik P (2007) Changes in organic—C, N, P and K and enzymatic activities in vermicompost of biodegradable organic wastes under liming and microbial inoculants. Bioresour Technol 98(13):2485–2494. doi:10.1016/j.biortech.2006.09.017
Samuel P, Ingmar P, Boubie G, Daniel L (2013) Trivalent chromium removal from aqueous solution using raw natural mixed clay from Burkina Faso. Int Res J Environ Sci 2(2):30–37
Singh J, Kalamdhad AS (2013) Reduction of bioavailability and leachability of heavy metals during vermicomposting of water hyacinth. Environ Sci Pllut Res 20:8974–8985. doi:10.1007/s11356-013-1848-x
Singh WR, Pankaj SK, Kalamdhad AS (2015) Reduction of bioavailability and leachability of heavy metals during agitated pile composting of Salvinia natans weed of Loktak Lake. Int J Recycl Org Waste Agricult 4:143–156. doi:10.1007/s40093-015-0094-2
Sinha RK, Herat S, Aarwal S, Asadi R, Carretero E (2002) Vermiculture and waste management: study of action of earthworms Eisenia fetida, Eudrilus eugeniae and Perionyx excavatus on biodegradation of some community wastes in India and Australia. Environmentalist 22:261–268
Sizmur T, Hodson ME (2009) Do earthworms impact metal mobility and availability in soil?—A review. Environ Pollut 157(7):1981–1989. doi:10.1016/j.envpol.2009.02.029
Sonowal P, Meena Khwairakpam M, Kalamdhad AS (2014) Stability analysis of dewatered sludge of pulp and paper mill during vermicomposting. Waste Biomass Valor 5:19–26. doi:10.1007/s12649-013-9225-z
Subbarao GV, Ito O, Berry WL, Wheeler RM (2003) Sodium—a functional plant nutrient. Crit Rev Plant Sci 22(5):391–416. doi:10.1080/07352680390243495
Suthar S (2007) Nutrient changes and biodynamics of epigeic earthworm Perionyx excavatus (Perrier) during recycling of some agriculture wastes. Bioresour Technol 98(8):1608–1614. doi:10.1016/j.biortech.2006.06.001
Suthar S (2009) Vermistabilization of municipal sewage sludge amended with sugarcane trash using epigeic Eisenia fetida (Oligochaeta). J Hazard Mater 163(1):199–206. doi:10.1016/j.jhazmat.2008.06.106
Suthar S (2010) Recycling of agro-industrial sludge through vermitechnology. Ecol Eng 36(8):1028–1036. doi:10.1016/j.ecoleng.2010.04.015
Suthar S, Sajwan P, Kumar K (2014) Vermiremediation of heavy metals in wastewater sludge from paper and pulp industry using earthworm Eisenia fetida. Ecotoxicol Environ Saf 109:177–184. doi:10.1016/j.ecoenv.2014.07.030
Tognetti C, Laos F, Mazzarino MJ, Hernandez MT (2005) Composting vs. vermicomposting: a comparison of end product quality. Comp Sci Util 13(1):6–13. doi:10.1080/1065657X.2005.10702212
Trisal CL, Manihar Th (2004) The Atlas of Loktak Lake. Wetland International, Canada and Loktak Development Authority, India
USEPA (1992) Method 1311–toxicity characteristic leaching procedure (TCLP) In: SW-846 Test Methods for Evaluating Solid Waste, Physical/Chemical Methods. US Environment Protection Agency http://www.epa.gov/solidwaste/hazard/testmethods/sw846/index.htm. Accessed 21 Jan 2014
Versteegh EAA, Black S, Hodson ME (2014) Environmental controls on the production of calcium carbonate by earthworms. Soil Biol Biochem 70:159–161
Vig AP, Singh J, Wani SH, Dhaliwal SS (2011) Vermicomposting of tannery sludge mixed with cattle dung into valuable manure using earthworm Eisenia fetida (Savigny). Bioresour Technol 102(17):7941–7945. doi:10.1016/j.biortech.2011.05.056
Walter I, Martinez F, Cala V (2006) Heavy metal speciation and phytotoxic effects of three representative sewage sludge for agricultural uses. Environ Pollut 139(3):507–514. doi:10.1016/j.envpol.2005.05.020
Wang P, Changa CM, Watson ME, Dick WA, Chen Y, Hoitink HAJ (2004) Maturity indicators for composted dairy and pig manures. Soil Biol and Biochem 36(5):767–776. doi:10.1016/j.soilbio.2003.12.012
Wong JWC, Selvam A (2006) Speciation of heavy metals during co-composting of sewage sludge with lime. Chemosphere 63(6):980–986. doi:10.1016/j.chemosphere.2005.08.045
Yadav A, Garg VK (2011) Vermicomposting—an effective tool for the management of invasive weed Parthenium hysterophorus. Bioresour Technol 102(10):5891–5895. doi:10.1016/j.biortech.2011.02.062
Acknowledgments
The authors gratefully acknowledge the financial support of the Council of Scientific and Industrial Research, Government of India, New Delhi (Grant No. 24(0319)/12/EMR-II dt. 07/05/2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Singh, W.R., Kalamdhad, A.S. Transformation of nutrients and heavy metals during vermicomposting of the invasive green weed Salvinia natans using Eisenia fetida . Int J Recycl Org Waste Agricult 5, 205–220 (2016). https://doi.org/10.1007/s40093-016-0129-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-016-0129-3