Abstract
Oxides of copper have been investigated for decades due to their unique semiconductor and optical properties. The review of literature revealed that very few reports are available on the synthesis of copper oxide nanoparticles using microorganisms and plant extracts. In this paper, we have reported the synthesis of copper oxide nanoparticles (CuO) using tea leaf and coffee powder extracts under microwave irradiations. The synthesis was carried out by irradiating metal salt and the extracts of tea and coffee in 1:3 ratio in a microwave at 540 W for 7–8 min. The synthesized nanoparticles were characterized by Scanning electron microscope, X-ray diffraction, UV–visible spectroscopy and Fourier transform infrared spectroscopy. The antibacterial activity of these nanoparticles was tested against six human pathogenic microbes. It was interesting to find that these nanoparticles possess remarkable antibacterial activity against two human pathogenic bacteria. Moreover, the use of environmentally benign materials for the synthesis of CuO nanoparticles offers numerous benefits of eco-friendliness and compatibility for pharmaceutical and other biomedical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of rapid and reliable processes for the preparation of nanosized metal particles has attracted significant attention due to their unusual size-dependent optical and electronic properties. Till now, a large number of physical, chemical and biological methods were available to synthesize different types of nanoparticles [1–4]. However, it is a fact that reproducibility and stability of the nanoparticles with controlled size are very difficult to achieve by popular chemical reduction methods. Synthesis of nanoparticles using plant extracts is quite novel and leads to true green chemistry at a very affordable cost [5–12].
Oxides of copper have been investigated for decades due to their unique semiconductor and optical properties [13–16]. Few studies have reported that copper nanoparticles exhibit excellent bactericidal properties [17–20]. Different methods have been available in the literature for the preparation of copper oxide nanoparticles, where flammable or corrosive reducing agent has been used such as titanium tetrachloride and hydrazine. Some wet chemical methods employed the toxic organic reactant such as ethylene glycol, while certain methods used additional reducing agent such as sodium tartrate [21–23]. In continuation of our earlier efforts for the synthesis of new carbon and metal nanoparticles for their applications in different fields [24–27], we have now reported the synthesis of nanosized copper oxide (CuO) via a green route, using tea leaf and coffee powder extracts. The main aim was that the nanoparticles should be water-dispersible and stable in aqueous media for prolonged periods, to be useful in biological applications. Although the exact mechanism for the synthesis of nanoparticles using plant extracts has not been devised, but it was suggested that different polyol components are responsible for the synthesis of nanoparticles. We have shown a possible mechanism about the formation of CuO nanoparticles from copper (II) ion. Further, to realize the potential of CuO nanoparticles as antimicrobial agents, these were tested against six human pathogenic bacteria.
Experimental procedure
Preparation of tea leaf and coffee powder extract
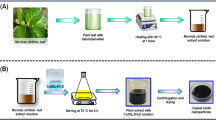
Two extracts were used to produce CuO nanoparticles. 20 g fine grounded powder of tea leaf and coffee powder were separately dissolved in 200 ml of water and boiled for around 3 h. After cooling at room temperature, these were centrifuged for 15 min and filtered. The filtrates were stored at 5–10 °C for further experiments.
Synthesis of CuO nanoparticles using tea leaf extract
Copper nitrate and tea leaf extract were taken in 1:3 ratio and the solution was subjected to microwave heating at 540 W which produced dark brown precipitate after 7–8 min. The precipitate was centrifuged, filtered and then dried in hot air oven for 4–5 h. The probable mechanism of the formation of CuO nanoparticles has been shown in Scheme 1.
Synthesis of CuO nanoparticles using coffee extract
Copper nitrate and coffee powder extract taken in 1:3 ratio were subjected to microwave heating at 540 W for 7–8 min which produced a brownish-black precipitate at the end. The precipitate was centrifuged, filtered and then dried in hot air oven for 4–5 h.
Antibacterial activity of CuO nanoparticles
The antibacterial activity of CuO nanoparticles was tested against six human pathogenic microbes viz., Shigella dysenteriae 1, Vibrio cholerae non.0139 (L4), Vibrio cholerae non.0139 (CSK6669), Streptococcuspneumoniae, Staphylococcus aureus and Escherichia coli. The antimicrobial assay was done using the disc diffusion method. Solutions of CuO nanoparticles were prepared in water having different concentrations of 200, 100, 50 and 1 μg/disc. The test microorganisms (freshly cultured in Luria Britani broth) were seeded into respective medium (agar–agar) by spreading 40 μl of each strain, using spread plate method. The autoclaved paper discs (5 mm in diameter and 0.4 mm in thickness) were then placed in petri dishes of 100 mm diameter, containing test microorganisms in agar media, followed by addition of different concentrations of solutions in each disc. Water was applied as control for each solution. The plates were then incubated at 37 °C for overnight. The antimicrobial activities were evaluated by measuring the zone of inhibition in millimeters (mm). The mean value of the readings was recorded after repeating each experiment three times.
Results and discussion
The formation of CuO nanoparticles from tea leaf extract was initially monitored by color change (Fig. 1). The SEM image clearly indicated the formation of spherical copper oxide nanoparticles with a size of 50–100 nm (Fig. 2). The UV–visible spectrum of CuO showed the absorption peak at 271 nm (Fig. 3) which was matched with the expected value of copper oxide nanoparticles [5]. Figure 4 shows the Fourier transform infrared spectroscopy (FTIR) peaks of CuO nanoparticles at 756, 1,070, 1,380, 1,600 and 3,420 cm−1. The peak at 1,070 cm−1 indicated the presence of C–O stretching frequency, whereas the geminal methyl group showed its presence at 1,380 cm−1. The peak at 1,600 cm−1 represented the unreacted ketone group suggesting the presence of flavonones adsorbed on the surface of CuO nanoparticles. This suggests that water-soluble organic moieties of tea were responsible for the synthesis of CuO. The FTIR spectrum also showed a broad absorption band at 3,420 cm−1 mainly for -OH groups present on the surface of the CuO nanostructures. The formation of CuO nanoparticles from coffee powder extract was initially observed through color change of the solution as shown in Fig. 5. Figure 6 shows the SEM image of CuO indicating the formation of spherical-shaped nanoparticles below 100 nm. The UV–visible spectrum of CuO nanoparticles showed a strong absorption peak at 269 nm (Fig. 7), matching with the literature value. The FTIR spectrum given in Fig. 8 again confirmed the formation of CuO nanoparticles in presence of coffee extract, showing peaks at 482, 1,080, 1,370, 1,620 and 3,440 cm−1. This has again indicated the secretion of some water-soluble organic components from coffee, which might have contributed in the formation of copper oxide nanoparticles via similar mechanism. The microwave irradiation accelerated the processes and the reactions were completed in very short period of time (7–8 min.). The reactions also gave products in absence of microwave heating but it took almost 4–5 h to complete the reaction. The possible mechanism is shown in the reaction Scheme 1. This gives strong evidence for the involvement of polyphenols in the rapid biosynthesis and for the stability of metallic nanoparticles in the aqueous medium [28]. It suggests that well-dispersed copper nanoparticles may have obtained through the reduction of Cu2+ using tea leaf and coffee extracts which contains epigallocatechin gallate (EGCG). EGCG is a highly water-soluble compound with strong polarity and it has acted as both reducing and capping agent. As illustrated in Scheme 1, EGCG served as a stable (electron + proton) donor during interactions. It was first converted into the radical ion “semihydro-EGCG” and then into dehydro-EGCG through oxidation. Dehydro-EGCG and EGCG together constituted the redox system which was sufficient to reduce Cu2+ to Cu(0). The lone pair of electrons in the polar groups of EGCG might have occupied the two sp orbits of the copper ion to form a complex compound. Thus, EGCG was capped with the copper ions to synthesize Cu(0) nanoparticles through reduction of Cu2+ inside the nanoscopic templates. In the presence of nanoscopic templates, small copper nanoparticles were initially formed which on further oxidation gave copper oxide nanoparticles. There is always a need of the development of resistant strains of bacteria which has increased the need for new antibiotics [29, 30]. To recognize the potential of CuO nanoparticles as antimicrobial agent, we have studied the antibacterial activity of synthesized CuO nanoparticles against six human pathogenic bacteria. Experimental data in the present study suggested that these nanoparticles have antibacterial activity against all the pathogenic microbial strains in different concentrations, in the range of 5–16 mm zone of inhibition. But, it showed remarkable antibacterial activity (12 mm inhibition zone with 200 μg/disc) against, S. dysenteriae 1 and Vibrio cholerae non.0139, as shown in Table 1. The antibacterial activity of CuO nanoparticles may be attributed to the fact that the size of the bacterial cells is usually in the micron range which have cellular membranes containing pores in nanometer range. These nanoparticles may have size less than the pore size of the bacteria and thus, they have easily crossed the cell membrane without any hindrance.
Conclusion
In this paper, we have reported for the first time, the use of tea leaf and coffee powder extracts for the synthesis of CuO nanoparticles using water as solvent under microwave irradiations. The CuO nanoparticles were found to be spherical in shape with a size of 50–100 nm. The synthesized nanoparticles showed remarkable antibacterial activity against two pathogenic bacteria and thus can be used as promising antibacterial agents in future. The use of environmentally benign materials like plant extracts, for the synthesis of nanoparticles offers numerous benefits of eco-friendliness and compatibility. Besides, the applications of such nanoparticles in pharmaceutical and other biomedical applications make this method potentially use for the large-scale synthesis of other inorganic nanomaterials.
References
Liu, J., Qiao, S.Z., Hu, Q.H.: Magnetic nanocomposites with mesoporous structures: synthesis and applications. Small 7, 425–443 (2011)
Luechinger, N.A., Grass, R.N., Athanassiou, E.K.: Bottom-up fabrication of metal/metal nanocomposites from nanoparticles of immiscible metals. Chem. Mater. 22, 155–160 (2010)
Mohanpuria, P., Rana, N.K., Yadav, S.K.: Biosynthesis of nanoparticle technological concepts and future applications. J. Nanopart. Res. 10, 507–517 (2008)
Honary, Soheyla, Barabadi, Hamed, Gharaei-Fathabad, Eshrat: Green synthesis of copper nanoparticles using penicillium aurantiogriseum, penicillium citrinum and penicillium citrinum and penicillium waksmani. Dig. J. Nanomater. Biostruct. 7, 999–1005 (2012)
Capek, I.: Preparation of metal nanoparticles in water-in-oil (w/o) microemulsions. Adv. Colloid Interface Sci. 110, 49–74 (2004)
Yin, B.S., Ma, H.Y., Wang, S.Y.: Electrochemical synthesis of silver nanoparticles under protection of poly(N-vinylpyrroli- done). J. Phys. Chem. B 107, 8898–8904 (2003)
Zhu, J.J., Liu, S.W., Palchik, O.: Shape-controlled synthesis of silver nanoparticles by pulse sonoelectrochemical methods. Langmuir 16, 6396–6399 (2000)
Vanaja, M., Gnanajobitha, G., Paulkumar, K., Rajeshkumar, S., Malrkodi, C., Annadurai, G.: Phytosynthesis of silver nanoparticles. J. Nanostruct. Chem. 3, 17 (2013)
Ghorbani, Hamid Raza: Biosynthesis of silver nanoparticles using Salmonella typhimurium. J. Nanostruct. Chem. 3, 43 (2013)
Mirjalili, Mohammad, Niloofar, Taghmari, Marjan, Mirjalili: Antibacterial properties of nano silver finish cellulose fabric. J. Nanostruct. Chem. 3, 43 (2013)
Gopinath, K., Gowri, S., Arumugam, A.: Phytosynthesis of silver nanoparticles using Pterocarpus santalinus leaf extract and their antibacterial properties. J. Nanostruct. Chem. 3, 68 (2013)
Abboud, Y., Eddahbi, A., El Bourari, A., Aitenneite, H., Brouzi, K., Mouslim, J.: Microwave-assisted approach for rapid and green phytosynthesis of silver nano particles using aqueous onion (Allium Cepa) extract and their antibacterial activity. J. Nanostruct. Chem. 3, 84 (2013)
Snoke, D.: Coherent exciton Waves. Science 273, 1351 (1996)
Briskman, R.N.: A study of electrodeposited cuprous oxide photovoltaic cells. Solar Energy Mater. Solar Cells 27, 361–368 (1992)
Li, X., Gao, H., Murphy, C.J.: Nanoindentation of Cu2O nanocubes. Nano Lett. 4, 1903–1907 (2004)
Liu, R., Oba, F., Bohannan, E.W.: Epitaxial electrodeposition of ordered Cu2O(110) nanostructures on InP(111). Chem. Mater. 17, 725–729 (2005)
Horiguchi, H.: Size-dependent antimicrobial properties of CuO nanoparticle against Gram-positive and -negative bacterial strains. Chem. Antimicrob. Agents 5, 46–59 (1980)
Ojas, M., Bhagat, M., Gopalakrishnan, C.: Ultrafine dispersed CuO nanoparticles and their antibacterial activity. J. Exp. Nanosci. 3, 185–193 (2008)
Li, B., Yu, S., Hwang, J.Y.: Synthesis of silver-treated bentonite: evaluation of its antibacterial properties. J. Miner. Mater. Charact. Eng. 1, 61–68 (2002)
Condorelli, G.G., Costanzo, I.L., Fragala, I.L.: A single photochemical route for the formation of both copper nanoparticles and patterned nanostructured films. J. Mater. Chem. 13, 2409–2411 (2003)
Yu, Y., Du, F.P., Yu, J.C.: One-dimensional shape-controlled preparation of porous Cu2O nano-whiskers by using CTAB as a template. J. Solid State Chem. 177, 4640–4647 (2004)
Huang, Lei, Peng, Feng, Yu, Hao: Diameter-controlling growth of solid-cored carbon nanofibers on a pulse plated iron nanocrystalline substrate in flames. Mater. Res. Bull. 43, 3397–3407 (2008)
Shin, Ho, Sun, Song, Jae, Yong, Yu, Jin: Template-assisted electrochemical synthesis of cuprous oxide nanowires. Mater. Lett. 63, 397–399 (2009)
Saha, Mitali, Das, Soma: Electrochemical studies of carbon nanotube obtained from coconut oil as non enzymatic glucose biosensor. Adv. Sci. Eng. Med. 5, 1–4 (2013)
Das, S., Saha, M.: Preparation of carbon nanosphere from bamboo and its use in water purification. Current Trends Technol. Sci. 2(1), 174–177 (2012)
Debbarma, Monica, Das, Soma, Saha, Mitali: Effect of reducing agents on the structure of zinc oxide under microwave irradiation. Adv. Manuf. 1, 183–186 (2013)
Sutradhar, P., Debnath, N., Saha, M.: Microwave-assisted rapid synthesis of alumina nanoparticles using tea, coffee and triphala extracts. Adv Manuf. (2013). doi:10.1007/s40436-013-0043-0
Xiong, J., Wang, Y., Xue, Q.: Synthesis of highly stable dispersions of nanosized copper particles using l-ascorbic acid. Green Chem. 13, 900–904 (2011)
Zakaria, Z.A., Sufian, A.S., Ramasamy, K.: In vitro antimicrobial activity of Muntingia calabura extracts and fractions. Afr. J. Microbiol. Res. 4, 304–307 (2010)
Eloff, J.N.: A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 64, 711–713 (1998)
Acknowledgments
Authors are grateful to Tripura University for antibacterial activity. Financial approval from CPRI, Bangalore is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sutradhar, P., Saha, M. & Maiti, D. Microwave synthesis of copper oxide nanoparticles using tea leaf and coffee powder extracts and its antibacterial activity. J Nanostruct Chem 4, 86 (2014). https://doi.org/10.1007/s40097-014-0086-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40097-014-0086-1