Abstract
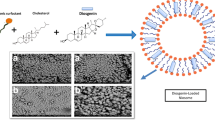
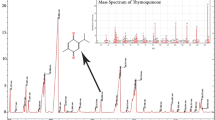
Phytochemicals like Lawsone have some drawbacks that stem from their poor solubility. Low solubility in aqueous mediums results in low bioavailability, poor permeability and instability of phytochemical compounds in biological environments. The aim of this study was to design nanoniosomes containing Lawsone (Law) using non-ionic surfactants and cholesterol. Niosomes were prepared by thin film hydration method (TFH). Then, they were loaded with Henna extract (HLaw) and standard Lawsone (SLaw), and two resulted formulations were compared. The henna extract was analyzed by mass gas chromatography. Size, zeta potential, polydispersity index (PDI) and morphology of the loaded formulations were evaluated by dynamic light scattering (DLS) and scanning electron spectroscopy (SEM). The incorporation and release rate of Law from niosome bilayers were evaluated by UV-Vis spectroscopy. In vitro experiments were carried out to evaluate antitumor activity in MCF-7 cell line. The results showed distinct spherical shapes and particle sizes were about 250 nm in diameter and have negative zeta potentials. Niosomes were stable at 4 °C for 2 months. Entrapment efficiently of both formulations was about 70% and showed a sustained release profile. In vitro study exhibited that using of niosome to encapsulating Law can significantly increase antitumor activity of formulation in MCF-7 cell line compared to Law solution (free Law). Thus, niosomes are a promising carrier system for delivery of phytochemical compounds that have poor solubility in biological fluids.

ᅟ





Similar content being viewed by others
Abbreviations
- Law:
-
Lawsone
- SLaw:
-
Standard Lawsone
- HLaw:
-
Henna Extract containing Lawsone
- Bare niosomes:
-
Niosomes without Law (Free niosomes)
- Nio/HLaw:
-
Niosome that loaded by HLaw
- Nio/Slaw:
-
Niosome that loaded by SLaw
References
Demarque DP, Fitts SMF, Boaretto AG, da Silva JCL, Vieira MC, Franco VN, et al. Optimization and technological development strategies of an antimicrobial extract from Achyrocline alata assisted by statistical design. PLoS One. 2015;10(2):e0118574.
Nicolaou K, Yang Z, Liu J, Ueno H, Nantermet P, Guy R, et al. Total synthesis of taxol. Nature. 1994;367(6464):630–4.
Castro FAV, Mariani D, Panek AD, Eleutherio ECA, Pereira MD. Cytotoxicity mechanism of two naphthoquinones (menadione and plumbagin) in Saccharomyces cerevisiae. PLoS One. 2008;3(12):e3999.
Rates SMK. Plants as source of drugs. Toxicon. 2001;39(5):603–13.
Preeti P. Pharmacognostical and phytochemical evaluation of Grewia asiatica Linn Tiliaceae fruit pulp and seed. International Journal of Pharmaceutical & Biological Archive (IJPBA). 2013;4(2).
Lauro M, De Simone F, Sansone F, Iannelli P, Aquino R. Preparations and release characteristics of naringin and naringenin gastro-resistant microparticles by spray-drying. J Drug Delivery Sci Technol. 2007;17(2):119–24.
Lauro M, Maggi L, Conte U, De Simone F, Aquino R. Rutin and quercetin gastro-resistant microparticles obtained by spray-drying technique. J Drug Delivery Sci Technol. 2005;15(5):363–9.
Sansone F, Picerno P, Mencherini T, Villecco F, D’ursi A, Aquino R, et al. Flavonoid microparticles by spray-drying: influence of enhancers of the dissolution rate on properties and stability. J Food Eng. 2011;103(2):188–96.
Sansone F, Rossi A, Del Gaudio P, De Simone F, Aquino RP, Lauro MR. Hesperidin gastroresistant microparticles by spray-drying: preparation, characterization, and dissolution profiles. AAPS PharmSciTech. 2009;10(2):391–401.
Ebrahimi AK, Barani M, Sheikhshoaie I. Fabrication of a new superparamagnetic metal-organic framework with core-shell nanocomposite structures: characterization, biocompatibility, and drug release study. Mater Sci Eng C 2018;(92):349-55.
Muhammad H, Muhammad S. The use of Lawsonia inermis Linn.(henna) in the management of burn wound infections. Afr J Biotechnol. 2005;4(9)
Hosein HKM, Zinab D. Phenolic compounds and antioxidant activity of henna leaves extracts (Lawsonia inermis). World J Dairy Food Sci. 2007;2(1):38–41.
McKelvey EM, Lomedico M, Lu K, Chadwick M, Loo TL. Dichloroallyl lawsone. Clin Pharmacol Ther. 1979;25(5part1):586–90.
Udapurkar P, Bhusnure O, Kamble S, Biyani K. Phyto-phospholipid complex vesicles for phytoconstituents and herbal extracts: a promising drug delivery system. Int J Herbal Med. 2016;4(5):14–20.
Singh RP, Narke R. Preparation and evaluation of phytosome of Lawsone. Int J Pharm Sci Res. 2015;6(12):5217.
Singh DK, Luqman S. Lawsonia inermis (L.): a perspective on anticancer potential of mehndi/henna. Biomedical Research and Therapy (BMRAT). 2014;1(04):112–20.
Knecht W, Henseling J, Löffler M. Kinetics of inhibition of human and rat dihydroorotate dehydrogenase by atovaquone, lawsone derivatives, brequinar sodium and polyporic acid. Chem Biol Interact. 2000;124(1):61–76.
El-Hag A, Al-Jabri A, Habbal O. Antimicrobial properties of Lawsonia inermis (henna): a review. Australian Journal of Medical Herbalism (AJMH). 2007;19(3):114.
Chaudhary G, Goyal S, Poonia P. Lawsonia inermis Linnaeus: a phytopharmacological review. International Journal of Pharmaceutical Sciences and Drug Research (IJPSDR). 2010;2(2):91–8.
LÓPEZ LÓPEZ LI, FLORES N, Daniel S, SILVA BELMARES SY, SÁENZ GALINDO A. Naphthoquinones: biological properties and synthesis of lawsone and derivatives-a structured review. Vitae. 2014;21(3):248–58.
Srinivas S, Kumar YA, Hemanth A, Anitha M. Preparation and evaluation of niosomes containing aceclofenac. Dig J Nanomater Bios. 2010;5(1):249–54.
Nematollahi MH, Pardakhty A, Torkzadeh-Mahanai M, Mehrabani M, Asadikaram G. Changes in physical and chemical properties of niosome membrane induced by cholesterol: a promising approach for niosome bilayer intervention. RSC Adv. 2017;7(78):49463–72.
James N, Coker R, Tomlinson D, Harris J, Gompels M, Pinching A, et al. Liposomal doxorubicin (Doxil): an effective new treatment for Kaposi's sarcoma in AIDS. Clin Oncol. 1994;6(5):294–6.
Bartelds R, Nematollahi MH, Pols T, Stuart MC, Pardakhty A, Asadikaram G, et al. Niosomes, an alternative for liposomal delivery. PLoS One. 2018;13(4):e0194179.
Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172(1–2):33–70.
Baillie A, Florence A, Hume L, Muirhead G, Rogerson A. The preparation and properties of niosomes—non-ionic surfactant vesicles. J Pharm Pharmacol. 1985;37(12):863–8.
Nematollahi MH, Torkzadeh-Mahanai M, Pardakhty A, Ebrahimi Meimand HA, Asadikaram G. Ternary complex of plasmid DNA with NLS-mu-mu protein and cationic niosome for biocompatible and efficient gene delivery: a comparative study with protamine and lipofectamine. Artif Cell Nanomed B. 2017:1–11. https://doi.org/10.1080/21691401.2017.1392316.
Sezgin-Bayindir Z, Yuksel N. Investigation of formulation variables and excipient interaction on the production of niosomes. AAPS PharmSciTech. 2012;13(3):826–35.
Brewer J, Alexander J. The adjuvant activity of non-ionic surfactant vesicles (niosomes) on the BALB/c humoral response to bovine serum albumin. Immunology. 1992;75(4):570–5.
Zimmermann GR, Lehar J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12(1):34–42.
Raja NR, Pillai G, Udupa N, Chandrashekar G. Anti-inflammatory activity of niosome encapsulated diclofenac sodium in arthritic rats. Indian J Pharm. 1994;26(1):46.
Luciani A, Olivier J-C, Clement O, Siauve N, Brillet P-Y, Bessoud B, et al. Glucose-receptor MR imaging of tumors: study in mice with PEGylated paramagnetic Niosomes 1. Radiology. 2004;231(1):135–42.
Schreier H, Bouwstra J. Liposomes and niosomes as topical drug carriers: dermal and transdermal drug delivery. J Control Release. 1994;30(1):1–15.
Aditya N, Aditya S, Yang H, Kim HW, Park SO, Ko S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015;173:7–13.
Ahmed K, Li Y, McClements DJ, Xiao H. Nanoemulsion-and emulsion-based delivery systems for curcumin: encapsulation and release properties. Food Chem. 2012;132(2):799–807.
Barras A, Mezzetti A, Richard A, Lazzaroni S, Roux S, Melnyk P, et al. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int J Pharm. 2009;379(2):270–7.
Puglia C, Lauro MR, Tirendi GG, Fassari GE, Carbone C, Bonina F, et al. Modern drug delivery strategies applied to natural active compounds. Expert Opin Drug Deliv. 2016:1–14.
MUSTAFA MAZ. DEVELOPMENT OF. NIOSOME CONTAINING ROASTED COFFEE RESIDUE EXTRACT FOR ANTIAGING PREPARATION: THAMMASAT UNIVERSITY; 2015.
Sebaaly C, Jraij A, Fessi H, Charcosset C, Greige-Gerges H. Preparation and characterization of clove essential oil-loaded liposomes. Food Chem. 2015;178:52–62.
Devendran G, Sivamani G. Phytochemical analysis of leaf extract of plant Costus spicatus by GCMS method. Journal of Drug Delivery and Therapeutics (JDDT). 2015;5(4):24–6.
Arunothayanun P, Uchegbu I, Craig D, Turton J, Florence A. In vitro/in vivo characterisation of polyhedral niosomes. Int J Pharm. 1999;183(1):57–61.
Hao Y-M, Ka L. Entrapment and release difference resulting from hydrogen bonding interactions in niosome. Int J Pharm. 2011;403(1):245–53.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
Stoline MR. The status of multiple comparisons: simultaneous estimation of all pairwise comparisons in one-way ANOVA designs. Am Stat. 1981;35(3):134–41.
Marianecci C, Rinaldi F, Di Marzio L, Mastriota M, Pieretti S, Celia C, et al. Ammonium glycyrrhizinate-loaded niosomes as a potential nanotherapeutic system for anti-inflammatory activity in murine models. Int J Nanomedicine. 2014;9:635.
Hans M, Lowman A. Biodegradable nanoparticles for drug delivery and targeting. Curr Opinion Solid State Mater Sci. 2002;6(4):319–27.
Müller R, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy: rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47(1):3–19.
Galante R, Rediguieri CF, Kikuchi IS, Vasquez PA, Colaço R, Serro AP, et al. About the sterilization of chitosan hydrogel nanoparticles. PLoS One. 2016;11(12):e0168862.
Harvey RD, Ara N, Heenan RK, Barlow DJ, Quinn PJ, Lawrence MJ. Stabilization of distearoylphosphatidylcholine lamellar phases in propylene glycol using cholesterol. Mol Pharm. 2013;10(12):4408–17.
Mahjub R, Dorkoosh FA, Amini M, Khoshayand MR, Rafiee-Tehrani M. Preparation, statistical optimization, and in vitro characterization of insulin nanoparticles composed of quaternized aromatic derivatives of chitosan. AAPS PharmSciTech. 2011;12(4):1407–19.
Hao Y, Zhao F, Li N, Yang Y. Li Ka. Studies on a high encapsulation of colchicine by a niosome system. Int J Pharm. 2002;244(1):73–80.
Owen SC, Chan DP, Shoichet MS. Polymeric micelle stability. Nano Today. 2012;7(1):53–65.
Soussan E, Cassel S, Blanzat M, Rico-Lattes I. Drug delivery by soft matter: matrix and vesicular carriers. Angew Chem Int Ed. 2009;48(2):274–88.
Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. Int J Pharm. 1998;168(2):221–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this study declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Barani, M., Mirzaei, M., Torkzadeh-Mahani, M. et al. Lawsone-loaded Niosome and its antitumor activity in MCF-7 breast Cancer cell line: a Nano-herbal treatment for Cancer. DARU J Pharm Sci 26, 11–17 (2018). https://doi.org/10.1007/s40199-018-0207-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-018-0207-3